| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
CDK7 (IC50 = 13.9 nM); CDK1 (IC50 = 96.9 nM); CDK2 (IC50 = 222 nM); CDK5 (IC50 = 134 nM); CDK9 (IC50 = 194 nM); CDK8 (IC50 = 6830 nM)
|
|---|---|
| 体外研究 (In Vitro) |
THZ2 有效抑制三阴性乳腺癌细胞生长,同时特异性靶向 CDK7,使 ER/PR+ 细胞不受影响。 THZ2 在低纳摩尔剂量下可有效抑制 TNBC 细胞的克隆生长,IC50 约为 10 nM。在三阴性乳腺癌细胞中,THZ2 会导致细胞凋亡,但在 ER/PR+ 乳腺癌细胞或正常人类细胞中则不然[1]。
|
| 体内研究 (In Vivo) |
THZ2 (10 mg/Kg) 具有抗肿瘤活性,并显着减缓小鼠肿瘤的生长速度。与媒介物处理的肿瘤相比,从用 THZ2 处理的小鼠分离的肿瘤组织表现出增殖减少和细胞凋亡增加,分别通过针对 Ki67 和裂解的 Caspase 3 的免疫染色证明。 THZ2 会降低 NOD-SCID 小鼠的体重,表明 THZ2 在这种特定小鼠品系中的耐受性可能较差[1]。
每天两次以10mg/kg的剂量腹腔注射THZ2治疗小鼠不会引起明显的毒性,如体重减轻或行为改变(数据未显示)。为了在原位异种移植物模型中测试THZ2是否对三阴性乳腺肿瘤有任何治疗作用,我们将三阴性乳腺癌细胞(MDA-MB-231)移植到裸鼠的乳腺脂肪垫中。当肿瘤达到约200 mm3时,用赋形剂或THZ2(10 mg/Kg)治疗小鼠。THZ2连续治疗25天不影响体重(图S2D),表明THZ2在裸鼠体内具有良好的耐受性。与对照肿瘤相比,用THZ2治疗的小鼠肿瘤的生长率显著降低(图2G),表明THZ2具有抗肿瘤活性。我们还收集了短期(50小时)或长期(25天)治疗后的肿瘤,发现急性和慢性THZ2暴露均显著降低了RNAPII在所有三个磷酸化位点(S2、S5和S7;图2H、S2E)的CTD磷酸化,表明CDK7在肿瘤细胞中有效靶向。与载体治疗的肿瘤相比,从用THZ2治疗的小鼠中分离的肿瘤组织增殖减少,凋亡增加,分别通过Ki67和切割的Caspase 3的免疫染色表明(图S2F)。总之,这些发现表明CDK7抑制剂能够有效地减少体内肿瘤细胞增殖并诱导细胞死亡。 研究人员进一步评估了CDK7抑制在两种独立的三阴性乳腺肿瘤患者来源的异种移植物(PDX)模型DFBC11-26和DFBC13-11中的抗肿瘤作用。这两种PDX模型都是从转移性TNBC患者中建立的,这些患者在多个化疗路线中取得了进展。将肿瘤碎片移植到NOD-SCID小鼠的乳腺脂肪垫中。我们在NOD-SCID小鼠中首次进行的THZ2实验导致体重减轻,这表明THZ2在这种特定的小鼠品系中可能耐受性较差。因此,我们继续在TNBC的PDX模型中使用THZ1。当肿瘤生长到平均大小约80mm3时,用THZ1治疗小鼠。尽管THZ1的药代动力学特性较差,但用这种药物治疗小鼠在两种患者衍生的肿瘤模型中都导致了肿瘤生长的实质性阻断(图2I和2J)。值得注意的是,THZ1治疗导致肿瘤细胞减少和疾病消退(图2J和2K)。对肿瘤组织的分析还表明,RNAPII的CTD磷酸化显著降低,并诱导PARP切割,这是凋亡细胞死亡的指标(图2L)。这些结果表明,CDK7抑制在患者来源的TNBC体内具有强大的抗肿瘤活性[1]。 |
| 酶活实验 |
THZ2与指定CDK结合效力的体外IC50。在不同浓度的THZ2存在下,用指定的CDK及其相关的细胞周期蛋白进行LanthaScreen Eu激酶结合分析(Invitrogen)。IC50值表明THZ2对CDK的ATP结合口袋的亲和力[1]。
|
| 细胞实验 |
在 96 孔板测定中,细胞以每孔 2000 个细胞的密度铺板,第二天,用不同浓度的 THZ1 或 THZ2 处理它们。 48 小时孵育期后,将细胞固定并用结晶紫染色。然后通过向每个孔中添加 10% 乙酸去除染色,并使用 750 nm 作为参考在 590 nm 处测量吸光度。
用增加浓度的THZ2处理7天的指定TNBC细胞系的细胞生长曲线。收获后,固定细胞,用结晶紫染色,然后提取染色液以定量增殖。 |
| 动物实验 |
Mice: A single 400 rad dose of γ-irradiation is given to naked mice (CrTac:NCr-Foxn1nu) six hours prior to cell transplantation. The fourth pair of mice's mammary fat pads are injected with 100 μL of breast cancer cells per site after the cells are extracted and resuscitated in 40% Matrigel-Basement Membrane Matrix, LDEV-free. Manual calipers are used to measure tumors in two dimensions. The formula for calculating tumor volume is V=0.5× length× width× width. THZ2 (3 mg/mL, prepared in vehicle solutions) at a dose of 10 mg/kg intraperitoneally twice daily is administered to animals with established tumors (mean tumor volume of approximately 200 mm3), which are randomly divided into two groups and treated with vehicle (10% DMSO in D5W, 5% dextrose in water). Tumor volume is measured every two to three days. After being harvested, tumors are cut in half. One half is immediately snap frozen in liquid nitrogen for immunoblotting, and the other half is fixed in formalin for one night before being examined histopathologically and then in 70% ethanol.
Stability of THZ1 and THZ2 in vivo. Mice were administered by tail vein injection of a single dose of THZ1 or THZ2, and blood samples were collected at different timepoints. Concentrations of THZ1 and THZ2 in plasma samples were determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS) approach.[1] |
| 药代性质 (ADME/PK) |
Despite the high anti-proliferative potency of THZ1 in primary TNBC cells, the stability of THZ1 in vivo (T1/2 – 45 min in mouse plasma) limits its utility for in vivo investigations. We therefore modified the structure of THZ1 by altering the regiochemistry of the acrylamide on THZ1 from 4-acrylamide-benzamide to 3-acrylamide-benzamide, giving rise to an analogue THZ2 (Figure 2A). THZ2 had significantly improved pharmacokinetic features, with a 5-fold improved half-life in vivo (Figure 2B). Similar to THZ1, THZ2 selectively targeted CDK7 (Figure 2C, Table S1) and potently inhibited the growth of triple-negative but not ER/PR+ breast cancer cells (Figure 2D, 2E). THZ2 at low nanomolar doses also efficiently suppressed the clonogenic growth of TNBC cells (IC50 of ~10 nM; Figure 2F, S2C). Like THZ1, THZ2 induced apoptotic cell death in triple-negative but not ER/PR+ breast cancer cells or normal human cells (Figure S2A), and did not cause an obvious alteration in cell cycle (Figure S2C). Therefore, we have identified an analogue of THZ2 with improved pharmacokinetic properties and comparable potency that we elected to use for further investigations. [1]
|
| 参考文献 | |
| 其他信息 |
Triple-negative breast cancer (TNBC) is a highly aggressive form of breast cancer that exhibits extremely high levels of genetic complexity and yet a relatively uniform transcriptional program. We postulate that TNBC might be highly dependent on uninterrupted transcription of a key set of genes within this gene expression program and might therefore be exceptionally sensitive to inhibitors of transcription. Utilizing kinase inhibitors and CRISPR/Cas9-mediated gene editing, we show here that triple-negative but not hormone receptor-positive breast cancer cells are exceptionally dependent on CDK7, a transcriptional cyclin-dependent kinase. TNBC cells are unique in their dependence on this transcriptional CDK and suffer apoptotic cell death upon CDK7 inhibition. An "Achilles cluster" of TNBC-specific genes is especially sensitive to CDK7 inhibition and frequently associated with super-enhancers. We conclude that CDK7 mediates transcriptional addiction to a vital cluster of genes in TNBC and CDK7 inhibition may be a useful therapy for this challenging cancer. [1]
|
| 分子式 |
C31H28CLN7O2
|
|---|---|
| 分子量 |
566.0527
|
| 精确质量 |
565.199
|
| 元素分析 |
C, 65.78; H, 4.99; Cl, 6.26; N, 17.32; O, 5.65
|
| CAS号 |
1604810-84-5
|
| 相关CAS号 |
1604810-84-5
|
| PubChem CID |
78357763
|
| 外观&性状 |
Light yellow to khaki solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 折射率 |
1.735
|
| LogP |
4.99
|
| tPSA |
115
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
41
|
| 分子复杂度/Complexity |
904
|
| 定义原子立体中心数目 |
0
|
| SMILES |
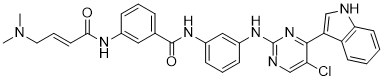
ClC1=C([H])N=C(N([H])C2C([H])=C([H])C([H])=C(C=2[H])N([H])C(C2C([H])=C([H])C([H])=C(C=2[H])N([H])C(/C(/[H])=C(\[H])/C([H])([H])N(C([H])([H])[H])C([H])([H])[H])=O)=O)N=C1C1=C([H])N([H])C2=C([H])C([H])=C([H])C([H])=C12
|
| InChi Key |
FONRCZUZCHXWBD-VGOFMYFVSA-N
|
| InChi Code |
InChI=1S/C31H28ClN7O2/c1-39(2)15-7-14-28(40)35-21-9-5-8-20(16-21)30(41)36-22-10-6-11-23(17-22)37-31-34-19-26(32)29(38-31)25-18-33-27-13-4-3-12-24(25)27/h3-14,16-19,33H,15H2,1-2H3,(H,35,40)(H,36,41)(H,34,37,38)/b14-7+
|
| 化学名 |
N-[3-[[5-chloro-4-(1H-indol-3-yl)pyrimidin-2-yl]amino]phenyl]-3-[[(E)-4-(dimethylamino)but-2-enoyl]amino]benzamide
|
| 别名 |
THZ-2; THZ 2; 1604810-84-5; N-[3-[[5-chloro-4-(1H-indol-3-yl)pyrimidin-2-yl]amino]phenyl]-3-[[(E)-4-(dimethylamino)but-2-enoyl]amino]benzamide; CHEMBL4303287; (E)-N-(3-((5-chloro-4-(1H-indol-3-yl)pyrimidin-2-yl)amino)phenyl)-3-(4-(dimethylamino)but-2-enamido)benzamide; THZ2
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 100 mg/mL (~176.7 mM)
Ethanol: ˂1 mg/mL (NaN mM) Water: ˂1 mg/mL (NaN mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.17 mg/mL (3.83 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 21.7 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.17 mg/mL (3.83 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 21.7 mg/mL澄清DMSO储备液加入900 μL玉米油中,混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7666 mL | 8.8331 mL | 17.6663 mL | |
| 5 mM | 0.3533 mL | 1.7666 mL | 3.5333 mL | |
| 10 mM | 0.1767 mL | 0.8833 mL | 1.7666 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。