| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
GSK-3β (IC50 = 5 nM); GSK-3β (IC50 = 60 nM)
|
|---|---|
| 体外研究 (In Vitro) |
Tideglusib (NP031112) 治疗完全消除了星形胶质细胞和小胶质细胞培养物中谷氨酸处理后 TNF- 和 COX-2 表达的诱导。由于星形胶质细胞和小胶质细胞 24 小时暴露于该 TDZD 对细胞活力没有影响,因此 NP031112 的这些影响并不是由细胞活力降低引起的[2]。
|
| 体内研究 (In Vivo) |
Tideglusib (NP031112) (50 mg/kg) 注射到大鼠海马中可显着减少红藻氨酸诱导的炎症(通过使用 T2 加权磁共振成像和神经胶质激活的水肿形成来测量),并且对海马受损区域具有神经保护作用。[2]
通过t2加权磁共振成像和神经胶质激活测量水肿形成情况,大鼠海马内共注射Tideglusib (NP031112)(一种更有效的噻二唑烷酮衍生物)可显著减少kainic酸诱导的炎症,并对海马受损区域具有神经保护作用。最后,通过与GW9662(2-氯-5-硝基苯胺)共处理,NP031112诱导的体外和体内神经保护作用显著减弱,GW9662是一种已知的核受体过氧化物酶体增殖体激活受体γ的拮抗剂,这表明NP031112的作用可以通过激活该受体来介导。因此,这些发现确定NP031112是治疗神经退行性疾病的潜在治疗剂。[2] |
| 酶活实验 |
将 55 μM 的 [35S]Tideglusib (207 Bq/nmol) 与 5 μM GSK-3β 在 315 μL 50 mM Tris-HCl(pH 7.5,含有 150 mM NaCl 和 0.1 mM EGTA)中于 25 °C 孵育 1 小时。添加 35 μL 相同缓冲液(含或不含 100 mM DTE)后,孵育再延长 30 分钟。最后,将每个原始样品的第三份 40 μL 等分试样与 10 μL 不含还原剂的变性电泳样品缓冲液混合,并将 35 μL 该混合物上样到 10% 聚丙烯酰胺凝胶上,进行 SDS-PAGE,然后进行荧光照相干燥的凝胶。最后,将每个原始样品的第三个 40 L 等分试样与 10 L 不含还原剂的变性电泳样品缓冲液混合,并将 35 L 该混合物上样到 10% 聚丙烯酰胺凝胶上,进行 SDS-PAGE,然后进行荧光成像干燥的凝胶。
对激酶面板抑制作用的评价[1] 在Invitrogen欧洲筛选中心评估了Tideglusib (NP031112)和hypothemycin对一组选定激酶的抑制活性。化合物在一组选定的激酶上以10 μm的单一浓度进行重复测试,使用Z ' -LYTETM技术在ATP和肽浓度接近其Km值时测量酶活性,除了MEK1, MEK2, p38α和JNK1,它们的ATP浓度为100 μm。在一些无法监测激酶(MEK3、NIK、TAK1-TAB1、MNK2、NLK和ZAK)活性的情况下,使用时间分辨FRET-based LanthaScreenTM技术测量了这些化合物取代已知atp竞争抑制剂荧光类似物结合的能力。关于测试的激酶的性质和在每种情况下获得的平均结果的详细信息列于补充表1中。 |
| 细胞实验 |
用指定浓度的药物处理细胞24小时。
|
| 动物实验 |
Rats; In this study, adult male Wistar rats (8–12 weeks old) are used. Rats (n≥5) are put into a stereotaxic machine. The hippocampus is given an injection of KA (1 μg in 2.5 μL l PBS) alone or in conjunction with Tideglusib (2 ng in 2.5 μL PBS). Animals in the control group are given vehicle injections and are the same age.
KA administration.[2] Adult male Wistar rats (8–12 weeks old) were used in this study. Adequate measures were taken to minimize pain or discomfort of animals. Experiments were performed in accordance with the European Communities Council, directive 86/609/EEC. Rats (n ≥ 5 per group) were anesthetized by intraperitoneal injection of ketamine (60 mg/kg) and Domtor (5 μg/kg) and placed into a stereotaxic apparatus. KA (1 μg in 2.5 μl PBS) alone or in combination with Tideglusib (NP031112) (2 ng in 2.5 μl PBS) was injected into the hippocampus [coordinates from bregma: posterior, −3.0 mm; lateral, −2.0 mm; depth, 3.5 mm; according to the atlas of Paxinos and Watson (1998)]. Control animals of the same age were injected with vehicle. Two groups of animals also received 0.7 μg of the PPARγ antagonist GW9662 (2-chloro-5-nitrobenzanilide), either alone or in combination with KA. Each injection was performed for >2.5 min using a micropump. The amounts of NP031112 and GW9662 used were calculated based on the in vitro results to reach active concentrations within the hippocampus. Lithium chloride (LiCl), a potent inhibitor of GSK-3β activity, was administered (40 mg/kg/d) by intraperitoneal injection to a further two groups of animals, either alone or in combination with KA. The rats were then housed individually to recover.[2] Seizures were induced by intraperitoneal administration of rats with KA (10 mg/kg) in PBS. Control animals received saline only. Behavioral analysis was monitored for a period of 3 h by trained observers blind to the treatment of the rats. The convulsive behavior was classified according to Racine (1972) and Sperk et al. (1985) as follows: stage 0, no changes; stage 0.5, wet dog shakes (WDS); stage 1, mouth and facial movements; stage 2, head nodding; stage 3, forelimbs clonus; stage 4, rearing; stage 5, rearing and falling; stage 6, death. Status epilepticus (SE) was defined as continuous behavioral seizure activity (stage 5) for ≥5 min. The number of WDS before SE was also examined. In trials using Tideglusib (NP031112), the TDZD was administered intragastrically (50 mg/kg) 1 h before KA injection. |
| 参考文献 |
|
| 其他信息 |
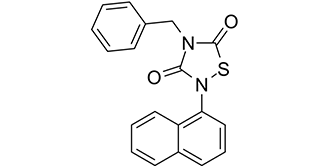
Tideglusib is a member of the class of thiadiazolidines that is 1,2,4-thiadiazolidine-3,5-dione which is substituted by a naphthalen-1-yl group at position 2 and by a benzyl group at position 4. It is a non-ATP competitive inhibitor of glycogen synthase kinase 3beta (GSK3beta) and has neuroprotective effects. Currently under clinical investigation for the treatment of Alzheimer's disease and progressive supranuclear palsy. It has a role as an EC 2.7.11.26 (tau-protein kinase) inhibitor, a neuroprotective agent, an anti-inflammatory agent and an apoptosis inducer. It is a member of naphthalenes, a member of benzenes and a thiadiazolidine.
Tideglusib is under the investigation for the development of treatments for Alzheimer's disease and for progressive supranuclear palsy. It is reported to be a potent anti-inflammatory and neuroprotective that is a non-ATP competitive inhibitor of glycogen synthase kinase 3 (GSK-3). Tideglusib is being developed by the Spanish pharmaceutic company Zeltia group and its current status is withdrawn for the treatment of Alzheimer's disease as of 2012. Drug Indication Tideglusib was initially formulated for the treatment of Alzheimer and progressive supranuclear palsy. The raising interest for the use of tideglusib comes from the significant upregulation of GSK-3 in the brain in patients with Alzheimer disease. Its function as a degradant of β-catenin, was also important, as it prevents the transcription of cell survival genes. All these factors have directed current research towards this kinase as a potential target. Alzheimer disease is the most prevalent form of dementia. The most accepted hypothesis to explain this disease is related to the presence of amyloid β, which triggers a cascade that will alter the Tau protein and provoke synaptic dysfunction and neuronal death. GSK-3 importance in the tissue repair pathway has also pointed out a novel application for tideglusib. Thus, it is also under the research for the natural repair treatment of deep caries lesions. Mechanism of Action GSK-3 is a proline/serine protein kinase that is ubiquitously expressed and involved in many cellular signaling pathways. From all its diverse functions, it plays a key role in Alzheimer's disease. This role is related to its link with β-amyloid and tau pathology. It has been suggested that aberrant Wnt or insulin signaling results in increased GSK-3 function. This kinase acts on γ-secretase producing the hyperphosphorylation of tau, the formation of neurofibrillary tangles and senile plaques. Tideglusib inhibits GSK-3 irreversibly by presenting a non-competitive inhibition pattern with respect to ATP. The binding of tideglusib seems to directly relate to the motif containing Cys199. Pharmacodynamics It is reported that tideglusib administration inhibits the activation of astrocytes and microglial cells, thus it presented a neuroprotective effect. It is known as well that the inactivation of GSK-3 protects against excitotoxicity. In pre-clinical trials, there have been reports of decrease Tau hyperphosphorylation, lower brain amyloid plaque load, learning and memory enhancement, prevention of neuronal loss and significant increases of the insulin growth factor 1 which is a potent neurotrophic peptide with therapeutic value.The reports in clinical trials have shown a trend in cognition increase of Alzheimer patients treated for 24 weeks. |
| 分子式 |
C19H14N2O2S
|
|---|---|
| 分子量 |
334.3917
|
| 精确质量 |
334.077
|
| 元素分析 |
C, 68.25; H, 4.22; N, 8.38; O, 9.57; S, 9.59
|
| CAS号 |
865854-05-3
|
| 相关CAS号 |
865854-05-3
|
| PubChem CID |
11313622
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
511.3±43.0 °C at 760 mmHg
|
| 熔点 |
148-150ºC
|
| 闪点 |
263.0±28.2 °C
|
| 蒸汽压 |
0.0±1.3 mmHg at 25°C
|
| 折射率 |
1.735
|
| LogP |
3.28
|
| tPSA |
72.24
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
492
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C1N(CC2C=CC=CC=2)C(=O)N(C2C3C(=CC=CC=3)C=CC=2)S1
|
| InChi Key |
PMJIHLSCWIDGMD-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C19H14N2O2S/c22-18-20(13-14-7-2-1-3-8-14)19(23)24-21(18)17-12-6-10-15-9-4-5-11-16(15)17/h1-12H,13H2
|
| 化学名 |
4-benzyl-2-naphthalen-1-yl-1,2,4-thiadiazolidine-3,5-dione
|
| 别名 |
Tideglusib; NP031112, NP-12; NP-12; NP031112; Tideglusib [INN]; 4-Benzyl-2-(naphthalen-1-yl)-1,2,4-thiadiazolidine-3,5-dione; NP-031112; tideglusibum; NP031112; NP 031112; NP-031112
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~66 mg/mL (~197.4 mM)
Water: <1 mg/mL Ethanol: <1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.48 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.48 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 4% DMSO+corn oil: 2.5mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9905 mL | 14.9526 mL | 29.9052 mL | |
| 5 mM | 0.5981 mL | 2.9905 mL | 5.9810 mL | |
| 10 mM | 0.2991 mL | 1.4953 mL | 2.9905 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Status | Interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05004129 | Recruiting | Drug: Tideglusib | Congenital Myotonic Dystrophy | AMO Pharma Limited | August 23, 2021 | Phase 2 Phase 3 |
| NCT05105958 | Not yet recruiting | Drug: Tideglusib | Amyotrophic Lateral Sclerosis | University Hospital, Geneva | December 1, 2025 | Phase 2 |
| NCT02858908 | Completed | Drug: Tideglusib | Myotonic Dystrophy 1 | AMO Pharma Limited | July 20, 2016 | Phase 2 |
| NCT01350362 | Completed | Drug: tideglusib Drug: Placebo |
Alzheimer's Disease | Noscira SA | April 2011 | Phase 2 |
| NCT00948259 | Completed | Drug: NP031112 Drug: Placebo |
Alzheimer´s Disease | Noscira SA | December 2008 | Phase 1 Phase 2 |
Inhibition of PI3K pathway signaling in cells. KPL-4 cells were treated with the indicated concentrations of CH5132799 for 2 hours. Clin Cancer Res, 2011, 17(10), 3272-3281. |
 Antitumor activity in mouse xenograft models of cell lines harboring genetic alterations, including PIK3CA mutations |
 Antitumor activity in combination with trastuzumab in the trastuzumab-insensitive model. |