| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Plasminogen activator inhibitor-1 (PAI-1) (IC50 = 6.95 μM)
|
|---|---|
| 体外研究 (In Vitro) |
根据对接实验,TM5275 附着在 PAI-1 的 A β-折叠 (s4A) 的第 4 链上。 TM5275 是一种选择性 PAI-1,浓度高达 100 μM 时不会干扰其他丝氨酸蛋白酶抑制剂/丝氨酸蛋白酶系统[1]。 TM5275 抑制 tPA-GFP-PAI-1 高分子量复合物的形成,因此在 20 和 100 μM 剂量下可显着延长 tPA-GFP 在 VEC 上的保留时间。 TM5275 加速纤溶酶原的时间依赖性积聚以及纤维蛋白凝块在表达 tPA-GFP 的细胞上及其周围的分解[2]。用 70-100 μM TM5275 处理的 ES-2 和 JHOC-9 细胞 72 小时后细胞活力降低。使用 100 μM TM5275,细胞生长被抑制 48-96 小时。当用 100 μM TM5275 处理时,与对照组相比,细胞培养基中的活性 PAI-1 显着减少。有人提出,TM5275 可能对 PAI-1 表达升高的卵巢癌具有抗增殖作用 [3]。
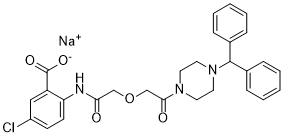
TM5275,5-氯-2-[({2-[4-(二苯甲基)哌嗪-1-基]-2-氧代乙氧基}乙酰基)氨基]苯甲酸酯(图1B),是通过对基于TM5007结构设计和合成的90多种化合物进行广泛的构效关系研究而发现的(图1A)。在考虑了体外PAI-1抑制活性和药代动力学研究(Tmax、Cmax、T½)后,TM5275最终被选为试验化合物(见下文)。 通过肽底物的tPA依赖性水解测量的TM5275的PAI-1抑制活性与TM5007和PAI-749相当:TM5275、TM5007、PAI-749的半最大抑制(IC50)值分别为6.95、5.60和8.37 μmol/L。 对PAI-1部分和TM5275进行对接模拟,以了解TM5275的作用机制。TM5275与PAI-1的Aβ-折叠(s4A)位置的链4结合。尽管TM5275和TM5007都在缝隙内与PAI-1的s4A片段结合,但仔细检查后发现它们的结合位点不同,如图2所示:TM5275的大体积二苯甲基不能容纳在TM5007的结合位点上,因此TM5275在s4A缝隙中明显移位。 在体外,TM5275(高达100 μmol/L)不会干扰其他丝氨酸蛋白酶系统(即α1抗胰蛋白酶/胰蛋白酶和α2抗纤溶酶/纤溶酶)。因此,其PAI-1抑制活性具有特异性。在十二烷基硫酸钠聚丙烯酰胺凝胶电泳上,当PAI-1与TM5275预孵育时,没有观察到与tPA形成的PAI-1共价复合物(数据未显示)。[1] TM5275在20和100μM的浓度下,通过抑制tPA-GFP-PAI-1高分子量复合物的形成,显著延长了tPA-GFP在VEC上的保留时间。TM5275增强了纤溶酶原的时间依赖性积聚,以及表达tPA-GFP的细胞上和周围纤维蛋白凝块的溶解。 TM5275的促纤维蛋白溶解作用通过PAI-1抑制导致的VEC表面tPA保留时间延长和纤溶酶生成增强得到了清楚的证明。[2] TM5275抑制纯化系统中rtPA和rPAI-1之间的高分子量复合物形成。 TM5275延长了tPA-GFP在VEC表面的保留时间。 TM5275抑制了EA.hy926细胞表面tPA和PAI-1之间的高分子量复合物形成,并减少了上清液中tPA-PAI-1复合物的量。 TM5275对VEC及其周围纤溶酶原积聚的影响。 TM5275对VEC纤维蛋白凝块溶解的疗效。 [2] TM5275对PAI-1的药理抑制降低卵巢癌症细胞的细胞增殖[3] TM5275是一种对PAI-1特异性的小分子抑制剂(图4A),已被开发为PAI-1相关疾病的治疗试剂。17,18我们研究了其作为靶向卵巢癌症细胞增殖的治疗试剂的潜力。用不同浓度的TM5275处理卵巢癌症细胞。70–100μM TM5275在ES-2和JHOC-9细胞中处理72小时后,细胞存活率降低,而其他细胞系对TM5275处理相对不敏感(图4B)。TM5275在卵巢癌症细胞系中的IC50值如表1所示。此外,在TM5275处理后的指定时间点测量细胞生长。从48小时到96小时,100μM TM5275抑制了细胞生长(图4C)。此外,与对照组相比,用100μM TM5275处理的细胞在细胞培养基中的活性PAI-1显著降低,证实了TM5275对PAI-1抑制的有效性(图4D)。这些结果表明,具有高表达PAI-1的卵巢癌症细胞,如ES-2和JHOC-9,易于受到TM5275的生长抑制。总之,TM5275对PAI-1的药理抑制作用可能在高表达PAI-1卵巢癌症中发挥抗增殖作用。 为了确定TM5275对ES-2细胞周期进程的影响,用或不用TM5275处理细胞24小时,并分析细胞周期分布。与载体处理相比,TM5275处理显著降低了G0/G1期细胞的百分比(52.0±1.5%至33.0±4.9%),增加了G2/M期细胞的比例(18.3±0.8%至27.7±2.0%)(图4E)。此外,用TM5275处理的细胞显示凋亡细胞百分比显著增加(图4F)。这些结果表明,PAI-1的药理学抑制诱导了卵巢癌症细胞中的G2/M细胞周期阻滞,并根据siRNA敲低PAI-1促进了细胞凋亡。 |
| 体内研究 (In Vivo) |
对于小鼠和大鼠,TM5275具有非常低的毒性和良好的药代动力学特征。在大鼠血栓形成模型中。与给予媒介物治疗的大鼠(72.5±2.0 mg)相比,以10和50 mg/kg剂量给予TM5275的大鼠具有显着更小的血凝块重量(分别为60.9±3.0和56.8±2.8 mg)。 TM5275(50 mg/kg)的抗血栓功效与参考抗血栓药物噻氯匹定(500 mg/kg)相当。剂量为10 mg/kg后,TM5275的血浆浓度达到17.5±5.2 μM。当 TM5275 (5 mg/kg) 与 tPA (0.3 mg/kg) 一起服用时,tPA (0.3 mg/kg) 的抗血栓作用大大增加,产生的益处与高剂量 tPA (3 mg/kg) 相当。 /千克)[1]。
大鼠血栓形成模型[1] 表1描述了TM5275在大鼠动静脉分流模型中的抗血栓作用。给药10和50的大鼠的血栓重量明显较低 TM5275(60.9±3.0和56.8±2.8)mg/kg mg)比赋形剂处理的大鼠(72.5±2.0 mg).高达300 需要mg/kg的TM5007才能达到50 同一模型中TM5275的浓度为mg/kg。TM5275(50mg/kg)的抗血栓作用与参考抗血栓化合物噻氯匹啶(500mg/kg)相当。TM5275血浆浓度达到17.5±5.2 μmol/L,剂量为10 mg/kg。 TM5275在大鼠FeCl3颈动脉血栓形成模型中的抗血栓作用如图3所示。TM5275和另一种标准抗血栓药物氯吡格雷以剂量依赖的方式证明了抗血栓作用(图3)。TM5275和氯吡格雷的最小有效剂量分别为1和3 mg/kg。它对应于TM5275的血浆浓度为4.9±3.6 μmol/L。正如预期的那样,氯吡格雷以剂量依赖的方式延长出血时间(图3)。相比之下,TM5275不影响出血时间,这是作为抗血栓药物的潜在益处。口服两小时后,TM5275(10mg/kg)不影响ADP和胶原蛋白诱导的血小板聚集(数据未显示)。因此,其抗血栓作用与对血小板的任何影响无关。 如图4所示,TM5275在同一型号中进一步与tPA结合。单独使用tPA(0.3mg/kg)不能提供显著的抗血栓作用。然而,TM5275(5 mg/kg)与tPA(0.3 mg/kg)联合使用显著增强了单独使用tPA(0.3mg/kg)的抗血栓作用,并提供了与高tPA剂量(3 mg/kg)相似的益处。联合治疗的出血时间与单独使用低剂量tPA(0.3mg/kg)的出血时间相似。 猴血栓模型[1] 在光化学诱导血栓形成的食蟹猴模型中评估了TM5275的抗血栓作用(表2)。TM5275的总闭塞时间显著缩短(53.9±19.9 分钟)和氯吡格雷(39.4±25.8 与赋形剂组(119.0±17.4)相比 分钟)。TM5275血浆浓度达到18.9±3.7 TM5275组为μmol/L。 TM5275作为一种对出血时间没有影响的抗血栓药物,其益处已在非人灵长类动物中得到证实(表3)。给药50小时后 mg/kg(比有效剂量高5倍)的TM5275,出血时间仅稍长(146.7±3.3 与给药前相比(83.3±6.7秒) secs),而在10 mg/kg氯吡格雷组(>600秒)高于给药前观察值(113.3±8.8秒) 秒)。 |
| 酶活实验 |
体外PAI-1活性测定[1]
PAI-1抑制活性通过之前描述的显色测定法进行评估(Izuhara等人,2008)。调整培养基的成分以提高测定的灵敏度:0.15 摩尔/升氯化钠,50 mmol/L Tris-HCl pH8,0.2 mmol/L CHAPS、0.1%PEG-6000、1%二甲亚砜、5 nmol/L人活性PAI-1,2 nmol/L人2链tPA和0.2 终浓度为mmol/L的Spectrozyme tPA。以不同浓度加入受试化合物,并通过logit-log分析计算IC50。 PAI-1/tPA复合物对十二烷基硫酸钠聚丙烯酰胺凝胶电泳的影响[1] 在混合PAI-1、tPA和化合物的培养基中估计受试化合物对PAI-1/tPA复合物形成的影响。最终的组成包括100 mmol/L HEPES,pH 7.4,150 mmol/L氯化钠、0.05%吐温20、0.8%二甲亚砜、0.875 μmol/L PAI-1,0.7 μmol/L tPA和化合物(160μmol/L)。蛋白质通过十二烷基硫酸钠聚丙烯酰胺凝胶电泳分离,并通过考马斯染色进行可视化。 SDS聚丙烯酰胺凝胶电泳(SDS-PAGE)[2] 在纯化系统中使用SDS-PAGE评估了TM5275对tPA-PAI-1复合物形成的影响。在37°C下与浓度为0(单独溶剂)、20和100μM的TM5275在HBS中孵育10分钟后,rPAI-1(终浓度为250 nM)与rtPA(终浓度270 nM)在37°C下孵育30分钟。与样品缓冲液(非还原性)混合后,对混合物进行10%SDS-PAGE,并用考马斯亮蓝对蛋白质条带进行染色。 纤维蛋白自动造影[2] 为了评估TM5275对PAI-1在培养的EA.hy926细胞或上清液中与tPA形成高分子量复合物的能力的影响,通过纤维蛋白自体移植对tPA-PAI-1复合物和游离tPA的量进行了半定量。在37°C下,在0(单独溶剂)、20和100μM TM5275存在下孵育3小时后,收集表达tPA-GFP或不表达EA.hy926细胞的培养基,在3000×g下离心10分钟以去除细胞碎片,与SDS样品缓冲液混合,并进行10%SDS-PAGE。如前所述,在分离蛋白质条带后,通过富含纤溶酶原的纤维蛋白指示剂凝胶检测tPA依赖性活性[17]。 纤溶酶原积累分析[2] 在37°C下用100μMTM5275或HBS/3%BSA溶剂处理30分钟后,将表达tPA-GFP的细胞与含有plg-568(20 nM)的人纤溶酶原(0.5μM)一起孵育。然后每10分钟用配备有60X油浸物镜的共聚焦激光扫描显微镜分析细胞表面或周围plg-568的积聚,该物镜捕获了波长为570nm至670nm的plg-568荧光。在每个实验结束前,我们添加了2.5μg/mL的Cell Mask Deep Red质膜染色,以鉴定表达tPA-GFP的细胞的定位。我们在单个细胞周围创建了一个感兴趣区域(ROI),包括最基底焦平面处的细胞周区域,并使用FV10-ASW软件(Olympus)测量了荧光强度。由于ROI内的平均荧光强度在10分钟内呈线性增加,我们计算了荧光随时间增加的斜率,表示plg-568的时间依赖性累积,并将其称为dF-plg。 纤维蛋白凝块溶解成像[2] tPA-GFP转染的细胞与100μM TM5275或HBS/3%BSA溶剂在37°C下预孵育30分钟。然后通过在HBS/3%BSA中混合0.5μM的人纤溶酶原(含有20 nM plg-568、2 U/mL凝血酶和1 mg/mL的人纤维蛋白原(含有10μg/mL fbg-647),在细胞上形成纤维蛋白凝块。在VEC上形成纤维蛋白凝块后,我们开始使用FV1000通过自动选择的二向色镜和每种荧光染料的适当波长范围每10分钟收集一次图像。然后,我们使用FV10-ASW软件计算了来自单个随机选择的tPA-GFP表达细胞的裂解面积,该细胞位于培养皿底部上方约3μm的焦平面处。 |
| 细胞实验 |
细胞活力测定[3]
用CellTiter Glo发光细胞活力测定法评估细胞活力。细胞以1-2×103/孔的密度接种在96孔板上。用siRNA TM5275处理后,向每个孔中加入80μl CellTiter-Glo试剂,然后在轨道振荡器上混合平板内容物。在标准光度计上对发光进行定量。 细胞周期分析[3] 对ES-2细胞(1×106/100mm培养皿)进行适当的处理。将细胞胰蛋白酶化,并用冰冷的70%乙醇固定过夜。用PBS洗涤固定细胞,并在100μg/ml RNase存在下用50μg/ml碘化丙啶(PI)染色。使用流式细胞仪的FL3通道定量细胞荧光。使用FlowJo软件确定细胞周期分布。 膜联蛋白V染色[3] 对ES-2细胞(1×106/100mm培养皿)进行适当的处理。将细胞胰蛋白酶消化,用PBS洗涤,并用来自死细胞凋亡试剂盒的AlexaFluor-488偶联的膜联蛋白V和PI染色。使用流式细胞仪分析染色细胞。 半胱天冬酶测定[3] 通过Caspase-Glo 3/7或Caspase Glo 8检测试剂盒测定Caspase活化。42细胞以2×103/孔的密度接种在96孔板上,用siRNA转染。72小时后,加入80μl单独的Caspase-Glo试剂。在室温下孵育0.5小时后,在微孔板光度计上读取样品。 |
| 动物实验 |
Pharmacokinetic Studies [1]
TM5275, suspended in 0.5% carboxymethyl cellulose sodium salt (CMC) solution, was administered orally by gavage to male ICR mice (50 mg/kg), male Wistar rats (50 mg/kg), and male cynomolgus monkeys (1 mg/kg). Heparinized blood samples were collected from the vein before (0 h) and 1, 2, 6, and 24 h after oral drug administration. Plasma drug concentration was determined on a reverse-phase high-performance liquid chromatography. Maximum drug concentration time (Tmax), maximum drug concentration (Cmax), and drug half-life (T½) were then calculated. For the bioavailability (BA) study in monkeys, heparinized blood samples were collected from the vein before (0 h) and 0.5, 1, 2, 4, 6, 8, 24, 48, 72, 120, and 168 h after oral drug administration, and before (0 h) and 0.08, 0.25, 0.5, 1, 2, 4, 6, 8, 24, 48, 72, 120, and 168 h after intravenous drug injection. BA was calculated by noncompartment model analysis using WinNonlin Professional Software, version 5.01 (Pharsight Co., NC, USA). Toxicity [1] For the evaluation of acute toxicity, TM5275 (1000 mg/kg for mice and 2000 mg/kg for rats and monkeys), suspended in 0.5% CMC solution, was administered orally by gavage to male (n=5) and female (n=5) ICR mice (CLEA Japan Inc.), male (n=5) and female (n=5) Sprague–Dawley rats (Charles River Japan Inc., Kanagawa, Japan), and male cynomolgus monkeys (n=2) (Japan SLC). The animal's body weight was monitored once a week. Various organs underwent histological studies 2 weeks (mice) and 1 week (rats) after drug administration. For the evaluation of the subacute toxicity, three different doses of TM5275 (200, 600, and 2000 mg/kg/day) were administered for 2 weeks by gavage to male (n=5) and female (n=5) Sprague–Dawley rats and male cynomolgus monkeys (n=2) (Japan SLC). At the end of the study, blood glucose, total cholesterol, triglyceride, aspartate aminotransferase, alanine aminotransferase, creatinine, urea nitrogen, total protein, albumin, hemoglobin, red blood cells, and hematocrit levels as well as activated partial thromboplastin time and prothrombin time were assessed. Body weight was measured and urinary analysis performed. The following safety pharmacology core battery was used: a modified Irwin's test for the central nervous system in Sprague–Dawley rats dosing TM5275 up to 2 g/kg per os, and three cardiovascular tests. (1) QT interval in telemetry electrocardiogram recording in beagle dogs administered an oral dose of 2 g/kg of TM5275; (2) action potentials of guinea-pig right ventricular papillary muscles at a dose of 5 μmol/L of TM5275; and (3) hERGIkr current measured in stably transfected human embryonic kidney (HEK) 293 cells at a dose of 5 μmol/L of TM5275. Arteriovenous Shunt Thrombosis Rat Model [1] Thrombus formation in arteriovenous shunts was achieved in male CD rats by a previously described method (Morishima et al, 1997). Either TM5275 (10 and 50 mg/kg, n=9) or ticlopidine (500 mg/kg, n=6), suspended in 0.5% CMC solution, was administered orally by gavage 90 mins before the study. Control rats were administered only a 0.5% CMC solution (n=10). Blood was allowed to circulate through the shunt for 30 mins. The wet weight of the thrombus covering the silk thread was eventually measured. Ferric Chloride-Treated Carotid Artery Thrombosis Rat Model [1] Male Sprague–Dawley rats weighing 280 to 310 g were anesthetized with pentobarbital sodium (50 mg/kg, intraperitoneally) and fixed on a heating pad. During the experiment, rectal temperature was maintained at 38°C. The left common carotid artery was exposed, and a piece of filter paper (2.5 × 4.2 mm) was folded around it. The probe of a pulsed Doppler flowmeter was placed to measure the arterial blood flow. After obtention of a steady baseline flow, 2 μL of ferric chloride (FeCl3) saline solution (35% (w/w)) was added to the filter paper. Five minutes later, the filter paper was removed and the artery washed with saline. Blood flow in the common carotid artery was continuously monitored for 30 mins after FeCl3 saline exposure. Time to primary occlusion was calculated. Several concentrations of TM5275 (0.3, 1, 5 mg/kg) and clopidogrel (1, 3, 10 mg/kg), suspended in 0.5% CMC solution, were administered orally by gavage (n=8, each group) 2 h before FeCl3 exposure. After a 30 mins blood flow monitoring, a sphygmomanometer cuff was placed on the tail and inflated to 40 mm Hg. An incision was made with an animal lancet (Goldenrod, Medipoint Inc., Mineola, NY, USA) and, every 30 secs, a wick of filtration paper was inserted on the wound until no further staining was observed. Bleeding time was determined to the nearest 30 secs. If it lasted more than 10 mins, the experiment was discontinued. The benefits of combining TM5275 with tPA were further ascertained. Either TM5275 (5 mg/kg, n=10), tPA (0.3 or 3 mg/kg, n=10 each), or TM5275 (5 mg/kg) plus tPA (0.3 mg/kg) (n=10) were administered orally (TM5275) or intravenously (tPA). The experiments were performed along the conditions described above. Photochemically Induced Arterial Thrombosis Monkey Model [1] Three- to 4-year-old male cynomolgus monkeys weighing 2.8 to 3.5 kg underwent anesthesia by an intramuscular injection of 10 mg/kg ketamine hydrochloride followed by the intravenous injection of 25 mg/kg pentobarbital sodium. Animals were fixed on a heating pad, and rectal temperature was maintained at 36.5 to 37.5°C. The saphenous artery was exposed by a 2 cm incision and thrombosis was induced by a photochemical reaction according to the modified method of Umemura et al (1993). Briefly, the saphenous artery was irradiated with green light (wave length 540 nm, 900,000 lx) generated by a xenon lamp with a heat-absorbing filter and a green filter. Irradiation was directed by a 3-mm diameter optic fiber mounted on a micromanipulator. The probe of a pulsed Doppler flowmeter was placed on the saphenous artery to measure arterial blood flow. Once the baseline flow was steady, a 20 mins photo-irradiation was undertaken and a 6 mins intravenous rose bengal (20 mg/kg) injection initiated. TM5275 (10 mg/kg) or clopidogrel (10 mg/kg) suspended in 0.5% CMC solution were administered by gavage (n=6, each group) 2 h before photochemical thrombosis. Blood flow of the saphenous artery was monitored for 3 h after the start of photo-irradiation. In monkeys, in contrast with rodents, photochemically induced arterial thrombosis progressively reduced cerebral blood flow, and was followed by recanalization and eventually by rethrombosis, a sequence observed in stroke patients and called cyclical flow reduction (Maeda et al, 2005a, 2005b). Therefore, total occlusion time was calculated during the experiment. Bleeding time was measured in monkeys previously acclimated to chair restraint during repeated training sessions several times before the experiment. A sphygmomanometer cuff, placed on the upper leg of conscious monkeys fixed to a monkey chair, was inflated to 40 mm Hg. TM5275 (50 mg/kg) or clopidogrel (10 mg/kg) suspended in 0.5% CMC solution were administered orally by gavage (n=3, each group) 2 h before the study. Bleeding was produced inside the lower leg by a Micro Lancet with a 21-gauge needle. Every 10 sec, a wick of filtration paper was placed on the wound until no further staining was observed. Bleeding time was determined to the nearest 10 secs. Maximum observation period was 10 mins. |
| 药代性质 (ADME/PK) |
Pharmacokinetics [1]
The pharmacokinetics of TM5275 improved significantly when compared with that of TM5007. An oral dose of 50 mg/kg of TM5275, administered in rats, yields calculated plasma Tmax, Cmax, and T½ of 2 h, 34 μmol/L, and 2.5 h, respectively, versus 18 h, 8.8 μmol/L, and 124 h, respectively, in rats administered the same dose of TM5007. TM5275 thus increases Cmax fourfold, and markedly shortens both Tmax and T½. In mice, an oral dose of 50 mg/kg of TM5275 yields the following values for these parameters: 1 h, 6.9 μmol/L, and 6.5 h, respectively. In monkeys, an oral dose of 1 mg/kg of TM5275 yields Tmax, Cmax, and T½ values of 6 h, 10.5 μmol/L, and 114.7 h, respectively. Bioavailability of TM5275 reaches 96% in monkeys. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity [1]
Acute toxicity has been evaluated in vivo. A single dose of TM5275 of 1000 mg/kg in mice and 2000 mg/kg in rats and monkeys elicited no symptoms after 2 weeks in the former group and after 1 week in the latter two groups. Body weight and the histology of various organs are not modified. Subacute toxicity has been assessed in rats and monkeys administered daily three different doses of TM5275 (200, 600, and 2000 mg/kg/day) for 2 weeks. Body weight and the histology of various organs are not modified. No abnormality is noted in the biochemistry of plasma and urine, including activated partial thromboplastin time, prothrombin time, and red blood cell count. In safety pharmacology studies, TM5275 does not modify tests of the central nervous system (a modified Irwin's test in rats) or of the cardiovascular system: (1) QT interval in electrocardiogram recording in dogs; (2) action potentials of guinea-pig right ventricular papillary muscles; and (3) hERGIkr current in HEK293 cells. |
| 参考文献 |
|
| 其他信息 |
Inhibition of plasminogen activator inhibitor (PAI)-1 is useful to treat several disorders including thrombosis. An inhibitor of PAI-1 (TM5275) was newly identified by an extensive study of structure-activity relationship based on a lead compound (TM5007) which was obtained through virtual screening by docking simulations. Its antithrombotic efficacy and adverse effects were tested in vivo in rats and nonhuman primates (cynomolgus monkey). TM5275, administered orally in rats (1 to 10 mg/kg), has an antithrombotic effect equivalent to that of ticlopidine (500 mg/kg) in an arterial venous shunt thrombosis model and to that of clopidogrel (3 mg/kg) in a ferric chloride-treated carotid artery thrombosis model. TM5275 does not modify activated partial thromboplastin time and prothrombin time or platelet activity and does not prolong bleeding time. Combined with tissue plasminogen activator, TM5275 improves the latter's therapeutic efficacy and reduces its adverse effect. Administered to a monkey model of photochemical induced arterial thrombosis, TM5275 (10 mg/kg) has the same antithrombotic effect as clopidogrel (10 mg/kg), without enhanced bleeding. This study documents the antithrombotic benefits of a novel, more powerful, PAI-1 inhibitor in rats and, for the first time, in nonhuman primates. These effects are obtained without adverse effect on bleeding time. [1]
Introduction: Elevated plasminogen activator inhibitor-1 (PAI-1) reduces fibrinolytic potential in plasma, contributing to thrombotic disease. Thus, inhibiting PAI-1 activity is clinically desirable. We recently demonstrated that tissue plasminogen activator (tPA) remains on the surface of vascular endothelial cells (VECs) after secretion in a heavy-chain dependent manner, which is essential for high fibrinolytic activity on the surface of VECs, and that PAI-1 dissociates retained tPA from the cell surface as a result of high-molecular weight complex formation. Based on the model whereby amounts of tPA and its equilibrium with PAI-1 dynamically change after exocytosis, we examined how TM5275, a newly synthesized small molecule PAI-1 inhibitor, modulated tPA retention and VEC surface-derived fibrinolytic activity using microscopic techniques. Materials and methods: The effects of TM5275 on the kinetics of the secretion and retention of green fluorescent protein (GFP)-tagged tPA (tPA-GFP) on VECs were analyzed using total internal reflection fluorescence microscopy. The effects of TM5275 on the generation of plasmin activity were evaluated by both plasminogen accumulation and fibrin clot lysis on tPA-GFP-expressing VECs using confocal laser scanning microscopy. Results: TM5275 at concentrations of 20 and 100 μM significantly prolonged the retention of tPA-GFP on VECs by inhibiting tPA-GFP-PAI-1 high-molecular-weight complex formation. TM5275 enhanced the time-dependent accumulation of plasminogen as well as the dissolution of fibrin clots on and around the tPA-GFP-expressing cells. Conclusions: The profibrinolytic effects of TM5275 were clearly demonstrated by the prolongation of tPA retention and enhancement of plasmin generation on the VEC surface as a result of PAI-1 inhibition. Keywords: CLSM; HBS; HEPES-buffered solution; PAI-1; TIRF; TM5275; VEC; confocal laser scanning microscope; fibrinolysis; plasminogen activator inhibitor-1; plasminogen activator inhibitor-1 (PAI-1); plasminogen activator inhibitor-1 (PAI-1) inhibitor; tPA; tissue plasminogen activator; total internal reflection fluorescence; total internal reflection fluorescence (TIRF) microscopy; vascular endothelial cell.[2] Plasminogen activator inhibitor (PAI)-1 is predictive of poor outcome in several types of cancer. The present study investigated the biological role for PAI-1 in ovarian cancer and potential of targeted pharmacotherapeutics. In patients with ovarian cancer, PAI-1 mRNA expression in tumor tissues was positively correlated with poor prognosis. To determine the role of PAI-1 in cell proliferation in ovarian cancer, the effects of PAI-1 inhibition were examined in PAI-1-expressing ovarian cancer cells. PAI-1 knockdown by small interfering RNA resulted in significant suppression of cell growth accompanied with G2/M cell cycle arrest and intrinsic apoptosis. Similarly, treatment with the small molecule PAI-1 inhibitor TM5275 effectively blocked cell proliferation of ovarian cancer cells that highly express PAI-1. Together these results suggest that PAI-1 promotes cell growth in ovarian cancer. Interestingly, expression of PAI-1 was increased in ovarian clear cell carcinoma compared with that in serous tumors. Our results suggest that PAI-1 inhibition promotes cell cycle arrest and apoptosis in ovarian cancer and that PAI-1 inhibitors potentially represent a novel class of anti-tumor agents.[3] |
| 分子式 |
C28H27CLN3NAO5
|
|
|---|---|---|
| 分子量 |
543.98
|
|
| 精确质量 |
543.153
|
|
| 元素分析 |
C, 61.82; H, 5.00; Cl, 6.52; N, 7.72; Na, 4.23; O, 14.71
|
|
| CAS号 |
1103926-82-4
|
|
| 相关CAS号 |
1103928-13-7 (free acid);1103926-82-4 (sodium);
|
|
| PubChem CID |
53240409
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| LogP |
2.541
|
|
| tPSA |
102.01
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
9
|
|
| 重原子数目 |
38
|
|
| 分子复杂度/Complexity |
752
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
C1CN(CCN1C(C2=CC=CC=C2)C3=CC=CC=C3)C(=O)COCC(=O)NC4=C(C=C(C=C4)Cl)C(=O)[O-].[Na+]
|
|
| InChi Key |
JSHSGBIWNPQCQZ-UHFFFAOYSA-M
|
|
| InChi Code |
InChI=1S/C28H28ClN3O5.Na/c29-22-11-12-24(23(17-22)28(35)36)30-25(33)18-37-19-26(34)31-13-15-32(16-14-31)27(20-7-3-1-4-8-20)21-9-5-2-6-10-21;/h1-12,17,27H,13-16,18-19H2,(H,30,33)(H,35,36);/q;+1/p-1
|
|
| 化学名 |
sodium;2-[[2-[2-(4-benzhydrylpiperazin-1-yl)-2-oxoethoxy]acetyl]amino]-5-chlorobenzoate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.60 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.60 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.60 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8383 mL | 9.1915 mL | 18.3830 mL | |
| 5 mM | 0.3677 mL | 1.8383 mL | 3.6766 mL | |
| 10 mM | 0.1838 mL | 0.9192 mL | 1.8383 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
 Decreased cell viability in cancer cells treated with TM5275 and TM5441.PLoS One.2015 Jul 24;10(7):e0133786. |
|---|
 Treatment with TM5275 or TM5441 increases intrinsic apoptosis.PLoS One.2015 Jul 24;10(7):e0133786. |
 Increased apoptosis in cancer cells treated with TM5275 and TM5441.PLoS One.2015 Jul 24;10(7):e0133786. |
 Decreased proliferation in cancer cells treated with TM5275 and TM5441.PLoS One.2015 Jul 24;10(7):e0133786. |
|---|
 Pre-clinical activity of TM5441 in vivo.PLoS One.2015 Jul 24;10(7):e0133786. |
 TM5441 inhibits EC branching morphogenesis.PLoS One.2015 Jul 24;10(7):e0133786. |
 TM compounds improve kidney function and morphology in STZ-induced diabetic mice.PLoS One.2016 Jun 3;11(6):e0157012. |
|---|
 TM compounds inhibit kidney fibrosis in STZ-induced diabetic mice.PLoS One.2016 Jun 3;11(6):e0157012. |
 TM compounds inhibit kidney inflammation in STZ-induced diabetic mice.
TM compounds inhibit PAI-1-induced fibrotic and inflammatory responsesin vitro.PLoS One.2016 Jun 3;11(6):e0157012. |