| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following administration of a 5-mg oral dose of 14C-tolterodine solution to healthy volunteers, 77% of radioactivity was recovered in urine and 17% was recovered in feces in 7 days. 113 ± 26.7 L Metabolism / Metabolites Tolterodine has known human metabolites that include 5-Hydroxymethyl tolterodine and N-Dealkylated tolterodine. Biological Half-Life 1.9-3.7 hours |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In multiple randomized controlled trials of tolterodine in patients with overactive bladder syndrome, serum aminotransferase and alkaline phosphatase elevations were not common, arising in less than 1% of treated subjects and in a similar proportion of placebo recipients. In these clinical trials there were no reports of clinically apparent liver injury or jaundice. Since the approval of tolterodine in 1998 and its widescale use (with more than 1 million prescriptions filled yearly in the United States), there has been only one published case report of liver injury with symptoms and jaundice attributed to its use (Case 1). Thus, acute symptomatic liver injury due to tolterodine must be very rare, if it occurs at all. Likelihood score: D (possible, very rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of tolterodine during breastfeeding. Long-term use of tolterodine might reduce milk production or milk letdown. During long-term use, observe for signs of decreased lactation (e.g., insatiety, poor weight gain). ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Anticholinergics can inhibit lactation in animals, apparently by inhibiting growth hormone and oxytocin secretion. Anticholinergic drugs can also reduce serum prolactin in nonnursing women. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Protein Binding Approximately 96.3%. |
| 参考文献 |
|
| 其他信息 |
Tolterodine is a tertiary amine. It has a role as a muscarinic antagonist, a muscle relaxant and an antispasmodic drug. It is functionally related to a p-cresol.
Tolterodine is an antimuscarinic drug that is used to treat urinary incontinence. Tolterodine acts on M2 and M3 subtypes of muscarinic receptors. Tolterodine is a Cholinergic Muscarinic Antagonist. The mechanism of action of tolterodine is as a Cholinergic Muscarinic Antagonist. Tolterodine is an anticholinergic agent used to treat urinary incontinence and overactive bladder syndrome. Tolterodine therapy has not been associated liver enzyme elevations while only a single case report has been published of clinical apparent acute liver injury attributed to its use. Tolterodine Tartrate is the tartrate salt form of tolterodine, a benzhydryl compound and a muscarinic receptor antagonist possessing both antimuscarinic and antispasmodic properties. Both tolterodine and its active metabolite, 5-hydroxymethyltolterodine, competitively blocks muscarinic receptors, thereby inhibiting acetylcholine receptor binding. This antagonistic action results in an increase in residual urine, reflecting an incomplete emptying of the bladder, and a decrease in detrusor pressure, indicating an antimuscarinic action on the lower urinary tract.. The 5-hydroxymethyl metabolite appears to contribute significantly to the therapeutic effects. Tolterodine is a benzhydryl compound and a muscarinic receptor antagonist possessing both antimuscarinic and antispasmodic properties. Both tolterodine and its active metabolite, 5-hydroxymethyltolterodine, competitively blocks muscarinic receptors, thereby inhibiting acetylcholine binding. This antagonistic action results in an increase in residual urine, reflecting an incomplete emptying of the bladder, and a decrease in detrusor pressure, indicating an antimuscarinic action on the lower urinary tract. The 5-hydroxymethyl metabolite appears to contribute significantly to the therapeutic effects. An ANTIMUSCARINIC AGENT selective for the MUSCARINIC RECEPTORS of the BLADDER that is used in the treatment of URINARY INCONTINENCE and URINARY URGE INCONTINENCE. See also: Tolterodine Tartrate (has salt form). Drug Indication For the treatment of overactive bladder (with symptoms of urinary frequency, urgency, or urge incontinence). FDA Label Mechanism of Action Both tolterodine and its active metabolite, 5-hydroxymethyltolterodine, act as competitive antagonists at muscarinic receptors. This antagonism results in inhibition of bladder contraction, decrease in detrusor pressure, and an incomplete emptying of the bladder. Pharmacodynamics Tolterodine is a competitive muscarinic receptor antagonist. Both urinary bladder contraction and salivation are mediated via cholinergic muscarinic receptors. After oral administration, tolterodine is metabolized in the liver, resulting in the formation of the 5-hydroxymethyl derivative, a major pharmacologically active metabolite. The 5-hydroxymethyl metabolite, which exhibits an antimuscarinic activity similar to that of tolterodine, contributes significantly to the therapeutic effect. Both tolterodine and the 5-hydroxymethyl metabolite exhibit a high specificity for muscarinic receptors, since both show negligible activity or affinity for other neurotransmitter receptors and other potential cellular targets, such as calcium channels. Tolterodine has a pronounced effect on bladder function. The main effects of tolterodine are an increase in residual urine, reflecting an incomplete emptying of the bladder, and a decrease in detrusor pressure, consistent with an antimuscarinic action on the lower urinary tract. |
| 分子式 |
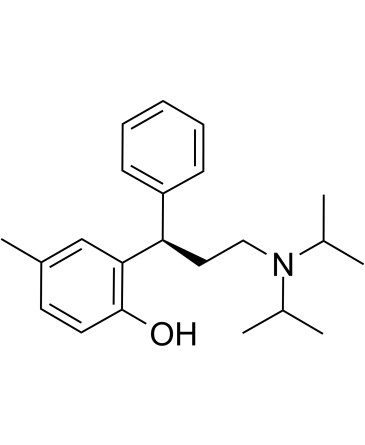
C22H31NO
|
|---|---|
| 分子量 |
325.48764
|
| 精确质量 |
325.24
|
| 元素分析 |
C, 81.18; H, 9.60; N, 4.30; O, 4.92
|
| CAS号 |
124937-51-5
|
| 相关CAS号 |
Tolterodine tartrate; 124937-52-6; Tolterodine-d14 hydrochloride; 1217645-16-3
|
| PubChem CID |
443879
|
| 外观&性状 |
Colorless to light yellow solid powder
|
| 密度 |
1.0±0.1 g/cm3
|
| 沸点 |
442.2±45.0 °C at 760 mmHg
|
| 闪点 |
192.1±27.4 °C
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
| 折射率 |
1.548
|
| LogP |
5.77
|
| tPSA |
23.47
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
340
|
| 定义原子立体中心数目 |
1
|
| SMILES |
CC(C)N(C(C)C)CC[C@H](C1=CC=CC=C1)C2=C(O)C=CC(C)=C2
|
| InChi Key |
OOGJQPCLVADCPB-HXUWFJFHSA-N
|
| InChi Code |
InChI=1S/C22H31NO/c1-16(2)23(17(3)4)14-13-20(19-9-7-6-8-10-19)21-15-18(5)11-12-22(21)24/h6-12,15-17,20,24H,13-14H2,1-5H3/t20-/m1/s1
|
| 化学名 |
2-[(1R)-3-[di(propan-2-yl)amino]-1-phenylpropyl]-4-methylphenol
|
| 别名 |
Detrusitol; Tolterodine; Detrol; PHA-686464B
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
Ethanol: ~120 mg/mL (~368.7 mM)
DMSO: ~100 mg/mL (~307.2 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (6.39 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (6.39 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (6.39 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0723 mL | 15.3615 mL | 30.7229 mL | |
| 5 mM | 0.6145 mL | 3.0723 mL | 6.1446 mL | |
| 10 mM | 0.3072 mL | 1.5361 mL | 3.0723 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Treatment Persistence Among Patients With Overactive Bladder: A Retrospective Secondary Data Analysis in Asia Oceania
CTID: NCT03602508
Phase: Status: Completed
Date: 2024-10-16