| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
MEK1 (IC50 = 2 nM); MEK2 (IC50 = 2 nM)
|
|---|---|
| 体外研究 (In Vitro) |
GSK1120212 的 IC50 范围为 0.92 nM 至 3.4 nM,无论 Raf 和 MEK 的同种型如何,都会抑制 MBP 的磷酸化。 c-Raf、B-Raf、ERK1 和 ERK2 不受 GSK1120212 激酶活性的抑制。此外,GSK1120212 并未显着抑制其他 98 种激酶。 GSK1120212 能有效抑制人结直肠癌细胞系。对 GSK1120212 敏感性最高的细胞的 IC50 值分别为 0.48 nM 和 0.52 nM,并且已知在 HT-29 和 COLO205 中具有组成型活性 B-Raf 突变体。 GSK1120212 在具有 K-Ras 突变的细胞系中的 IC50 范围为 2.2–174 nM,表明具有广泛的敏感性。相比之下,GSK1120212被发现对COLO320 DM细胞具有抗性,该细胞在B-Raf和K-Ras中均携带野生型基因。即使在 10 μM 下也是如此。所有敏感细胞系均经过 24 小时 GSK1120212 处理,导致细胞周期停滞在 G1 期。在大多数结直肠癌细胞系中,GSK1120212 治疗会导致 p15INK4b 和/或 p27KIP1 上调。在所有易受影响的细胞系中,GSK1120212 可以阻止 ERK 的组成型磷酸化。 HT-29 和 COLO205 细胞均经历 GSK1120212 诱导细胞凋亡;然而,COLO205 细胞比 HT-29 细胞更容易受到这种诱导。 [1] GSK1120212 阻断外周血单核细胞 (PBMC) 产生肿瘤坏死因子-α 和白细胞介素 6。 [2]
|
| 体内研究 (In Vivo) |
当每天口服一次 0.3 mg/kg 或 1 mg/kg 剂量的 GSK1120212,连续 14 天时,可以有效阻止 HT-29 异种移植物的生长。剂量为 1 mg/kg 时,肿瘤生长几乎完全停止。单次口服剂量1 mg/kg GSK1120212完全抑制已形成肿瘤组织中ERK1/2的磷酸化,治疗14天后,蛋白p15INK4b和p27KIP1的水平均升高。即使剂量为 0.3 mg/kg,COLO205 异种移植模型中也可以看到肿瘤消退。在接受 1 mg/kg 剂量的 6 只小鼠中,有 4 只的肿瘤完全消退,导致肿瘤体积无法测量。 [1] Lewis 大鼠或 DBA1/J 小鼠的佐剂诱导性关节炎 (AIA) 和 II 型胶原诱导性关节炎 (CIA) 几乎完全被 GSK1120212 0.1 mg/kg 的给药量所抑制。 [2]
|
| 酶活实验 |
将非磷酸化髓磷脂碱性蛋白 (MBP) 包被到 ELISA 板上,在不同浓度的 GSK1120212 存在下,将活性形式的 B-Raf/c-Raf 与未磷酸化的 MEK1/MEK2 和 EERRK2 结合。使用抗磷酸化 MBP 抗体,可以看到 MBP 磷酸化。
|
| 细胞实验 |
暴露于 GSK1120212 后,在 96 孔组织培养板中对指数生长的细胞进行 24 小时预培养。以磺基罗丹明 B 为主要成分的体外毒理学测定试剂盒可测量细胞生长。收集贴壁细胞和漂浮细胞并用 70% 乙醇固定,用于细胞凋亡测定。将细胞在 PBS 中洗涤,然后悬浮在 100 g/mL RNase 和 25 μg/mL 碘化丙啶 (PI) 中,并在黑暗中加热至 37°C 30 分钟。使用流式细胞仪 Cytomics FC500 或 Guava EasyCyte plus 可鉴定每个细胞的 DNA 含量。
|
| 动物实验 |
Mice: To create the A549 (human non-small cell lung carcinoma) model, tissue cultured cells are harvested aseptically and then digested with trypsin. Between 5×106 and 107 cells in 50% martigel are subcutaneously injected into female athymic mice (strain nu/nu). Before treatment, tumors are given one to four weeks to establish. The recommended doses of trametinib are given orally in amounts of 0.2 mL/20 g by weight. Vernier calipers are used every two weeks to measure tumors. When vehicle tumors reached a volume greater than 1000 mm3, antitumor activity was considered to be present. Tumor growth inhibition is defined as the percentage volume difference in tumor growth between the treated and control tumors at that time.
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following oral administration, trametinib is rapidly and readily absorbed. The absorption was examined in patients with solid tumours and BRAF V600 mutation-positive metastatic melanoma. Following the administration of trametinib tablets 0.125 mg (0.0625 times the approved recommended adult dosage) to 4 mg (2 times the approved recommended adult dosage) daily, both Cmax and AUC increased dose-proportionally. Intersubject variability in AUC and Cmax at steady state is 22% and 28%, respectively. Trametinib accumulates with daily repeat dosing with a mean accumulation ratio of 6.0 at 2 mg once daily dose. Steady-state was achieved by Day 15. The mean absolute bioavailability of trametinib is 72% for oral tablets and 81% for oral solution. The Tmax is 1.5 hours. A high-fat, high-calorie meal (approximately 1000 calories) decreased trametinib AUC by 24% and Cmax by 70%, and delayed Tmax by approximately four hours as compared with fasted conditions. Following oral administration of [14C]-trametinib, greater than 80% of excreted radioactivity was recovered in the feces while less than 20% of excreted radioactivity was recovered in the urine with less than 0.1% of the excreted dose as the parent molecule. The apparent volume of distribution (Vc/F) is 214 L. The apparent clearance is 4.9 L/h. Metabolism / Metabolites Trametinib predominantly undergoes deacetylation mediated by carboxylesterases (i.e., carboxylesterase 1b/c and 2) and other hydrolytic enzymes. The deacetylated metabolite may further be glucuronidated. _In vitro_ findings suggest that deacetylation may also be accompanied by mono-oxygenation, hydroxylation, and glucuronidation. CYP3A4-mediated oxidation is a minor pathway. Four metabolites (M1/2/3/4) have been characterized in patients with advanced cancers. _In vitro_, the M1 and M3 metabolites demonstrated approximately equal or 10-fold less potent phospho-MEK1-inhibiting activity than the parent compound. Following a single dose of [14C]-trametinib, approximately 50% of circulating radioactivity represented the parent compound. According to findings from metabolite profiling after repeat dosing of trametinib, unchanged parent drug accounted for greater than or equal to 75% of drug-related material in plasma. Biological Half-Life The estimated elimination half-life is 3.9 to 4.8 days. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In large clinical trials, abnormalities in routine liver tests were common with serum aminotransferase elevations occurring in 39% to 60% and alkaline phosphatase in 24% to 67% of patients treated with trametinib. However, elevations in ALT above 5 times the ULN were uncommon, occurring in 0% to 5% of patients and generally resolving rapidly with temporary discontinuation or dose adjustment. In the prelicensure controlled trials of trametinib with or without dabrafenib, no cases of clinically apparent acute liver injury or hepatic failure were reported. There have yet to be published cases of clinically apparent hepatotoxicity attributed to trametinib. However, it has been used for a short time only. Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of trametinib during breastfeeding. Because trametinib is 97% bound to plasma proteins, the amount in milk is likely to be low. However, its half-life is 3.9 to 4.8 days and it might accumulate in the infant. The manufacturer recommends that breastfeeding be discontinued during trametinib therapy and for 4 months after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Trametinib is 97.4% bound to human plasma proteins. |
| 参考文献 | |
| 其他信息 |
Trametinib dimethyl sulfoxide is an addition compound obtained by combining equimolar amounts of trametinib and dimethyl sulfoxide. Used for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations, and who have not received prior BRAF inhibitor treatment. It has a role as an antineoplastic agent and an EC 2.7.11.24 (mitogen-activated protein kinase) inhibitor. It contains a dimethyl sulfoxide and a trametinib.
Trametinib Dimethyl Sulfoxide is a dimethyl sulfoxide (DMSO) solvated form of trametinib, an orally bioavailable inhibitor of mitogen-activated protein kinase kinase (MAP2K; MAPK/ERK kinase; MEK) 1 and 2, with potential antineoplastic activity. Upon oral administration, trametinib specifically binds to and inhibits MEK 1 and 2, resulting in an inhibition of growth factor-mediated cell signaling and cellular proliferation in various cancers. MEK 1 and 2, dual specificity serine/threonine and tyrosine kinases often upregulated in various cancer cell types, play a key role in the activation of the RAS/RAF/MEK/ERK signaling pathway that regulates cell growth. See also: Trametinib (has active moiety). The MAPK pathway is one of the most important pathways for novel anticancer drug development. We performed high-throughput screening for compounds that induce expression of p15INK4b, and identified JTP-74057 (GSK1120212), which is being evaluated in ongoing phase I, II and III clinical trials. We characterized its antitumor activities in vitro and in vivo. JTP-74057 strongly inhibited MEK1/2 kinase activities, but did not inhibit another 98 kinase activities. Treatment by JTP-74057 resulted in growth inhibition accompanied with upregulation of p15INK4b and/or p27KIP1 in most of the colorectal cancer cell lines tested. Daily oral administration of JTP-74057 for 14 days suppressed tumor growth of HT-29 and COLO205 xenografts in nude mice. Notably, tumor regression was observed only in COLO205 xenografts, and COLO205 was much more sensitive to JTP-74057-induced apoptosis than HT-29 in vitro. Treatment with an Akt inhibitor enhanced the JTP-74057-induced apoptosis in HT-29 cells. Finally, JTP-74057 exhibited an additive or a synergistic effect in combination with the standard-of-care agents, 5-fluorouracil, oxaliplatin or SN-38. JTP-74057, a highly specific and potent MEK1/2 inhibitor, exerts favorable antitumor activities in vitro and in vivo. Sensitivity to JTP-74057-induced apoptosis may be an important factor for the estimation of in vivo efficacy, and sensitivity was enhanced by an Akt inhibitor. These results suggest the usefulness of JTP-74057 in therapeutic applications for colorectal cancer patients.[1] Objective and design: To examine the effects of a mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2-inhibitor, JTP-74057, on inflammatory arthritis development, and compare its anti-arthritic effect with leflunomide. Materials: Human, mouse, and rat peripheral blood mononuclear cells (PBMCs) were used. Lewis rats and DBA/1J mice were used for animal models. Treatment: JTP-74057 was tested between 0.1-100 nM in in-vitro studies. JTP-74057 (0.01-0.3 mg/kg) and leflunomide (2-10 mg/kg) were administered orally in vivo. Methods: PBMCs were stimulated with lipopolysaccharide. Adjuvant-induced arthritis (AIA) and type II collagen-induced arthritis (CIA) was induced in Lewis rats or DBA1/J mice, respectively. Results: JTP-74057 blocked tumor necrosis factor-α and interleukin-6 production from PBMCs. AIA and CIA development were suppressed almost completely by 0.1 mg/kg of JTP-74057 or 10 mg/kg of leflunomide. In the CIA, JTP-74057, but not leflunomide, suppressed collagen-reactive T-cell proliferation ex vivo, whereas leflunomide, but not JTP-74057, suppressed anti-collagen antibody production. Conclusions: JTP-74057 exerts potent anti-arthritic effects with a different profile from leflunomide, suggesting that JTP-74057 may be useful as a new therapeutic reagent in the treatment of rheumatoid arthritis.[2] |
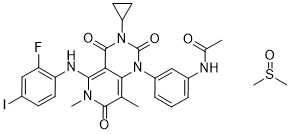
| 分子式 |
C28H29FIN5O5S
|
|
|---|---|---|
| 分子量 |
693.09
|
|
| 精确质量 |
693.091
|
|
| 元素分析 |
C, 48.49; H, 4.21; F, 2.74; I, 18.30; N, 10.10; O, 11.53; S, 4.62
|
|
| CAS号 |
1187431-43-1
|
|
| 相关CAS号 |
Trametinib;871700-17-3
|
|
| PubChem CID |
50992434
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| LogP |
5.523
|
|
| tPSA |
146.9
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
8
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
41
|
|
| 分子复杂度/Complexity |
1120
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
IC1C([H])=C([H])C(=C(C=1[H])F)N([H])C1=C2C(=C(C([H])([H])[H])C(N1C([H])([H])[H])=O)N(C1=C([H])C([H])=C([H])C(=C1[H])N([H])C(C([H])([H])[H])=O)C(N(C2=O)C1([H])C([H])([H])C1([H])[H])=O.S(C([H])([H])[H])(C([H])([H])[H])=O
|
|
| InChi Key |
OQUFJVRYDFIQBW-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C26H23FIN5O4.C2H6OS/c1-13-22-21(23(31(3)24(13)35)30-20-10-7-15(28)11-19(20)27)25(36)33(17-8-9-17)26(37)32(22)18-6-4-5-16(12-18)29-14(2)34;1-4(2)3/h4-7,10-12,17,30H,8-9H2,1-3H3,(H,29,34);1-2H3
|
|
| 化学名 |
N-[3-[3-cyclopropyl-5-(2-fluoro-4-iodoanilino)-6,8-dimethyl-2,4,7-trioxopyrido[4,3-d]pyrimidin-1-yl]phenyl]acetamide;methylsulfinylmethane
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (3.60 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (3.60 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (3.60 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4428 mL | 7.2141 mL | 14.4281 mL | |
| 5 mM | 0.2886 mL | 1.4428 mL | 2.8856 mL | |
| 10 mM | 0.1443 mL | 0.7214 mL | 1.4428 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01682083 | Completed | Drug: Dabrafenib Drug: Trametinib |
Melanoma | Novartis Pharmaceuticals | January 8, 2013 | Phase 3 |
| NCT02083354 | Completed | Drug: Dabrafenib Drug: Trametinib |
Cancer Melanoma |
Novartis Pharmaceuticals | March 18, 2014 | Phase 2 |