| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g | |||
| Other Sizes |
| 靶点 |
H1 receptor ( IC50 = 30 μM )
Histamine H1 receptor (H1R) [1] |
|---|---|
| 体外研究 (In Vitro) |
Tripelennamine 是叔胺 UDP-葡萄糖醛酸基转移酶的底物,可催化季铵连接的葡萄糖醛酸苷的形成。 Tripelennamine 通过竞争性和非竞争性抑制的混合作用,在人和兔肝微粒体中抑制 2-amino-1-methyl-6-苯基imidazo[4,5-b]pyridine (PhIP) 的葡萄糖醛酸化。与碱性溶液中完全中性分子记录的振动模式相比,在中性 pH 值下,氨烷基链被质子化,Tripelennamine 中氨基吡啶发色团的振动发生了变化。
|
| 体内研究 (In Vivo) |
盐酸曲苯那明 (iv) 会导致站立的马的中枢神经系统 (CNS) 兴奋,马变得非常警觉、烦躁和不舒服,表现为抬起头、收紧颈部肌肉、眼睛和耳朵过度快速运动,咬、喷鼻息、轻快地摆动尾巴、用前脚跺脚和抓爪。因此,在盐酸曲苯那敏处理后,站立的马的血红蛋白浓度显着增加。盐酸曲苯那敏 (iv) 显着增加站立马的混合静脉血 O2 张力和血红蛋白 O2 饱和度,以及动脉和混合静脉血 O2 含量,但站立马的动脉至混合静脉 O2 含量梯度不明显受到显着影响。在马和骆驼中给予盐酸曲苯那敏(0.5 mg/kg iv),最终消除半衰期分别为 2.39 和 2.08 小时,全身清除率为 0.97 和 0.84 L/h/kg。稳态分布容积为2.87和1.69 L/kg,两室药代动力学模型的中央室容积为1.75和1.06 L/kg。
4-6岁、体重450-550 kg的纯血马,运动前30分钟静脉注射盐酸曲吡那敏(Tripelennamine HCl)(0.5 mg/kg)。在亚最大强度运动(6 m/s,5分钟)和最大强度运动(直至疲劳)期间,与生理盐水对照组相比,该药物不影响动脉低氧血症(动脉血氧分压、血红蛋白氧饱和度),心率和血乳酸浓度也无变化[1] |
| 动物实验 |
0.5 mg/kg i.v.
Horses Thoroughbred horse exercise experiment: Healthy 4-6-year-old Thoroughbred horses (450-550 kg) were acclimated to treadmill exercise for 2 weeks. Tripelennamine HCl was dissolved in physiological saline and administered via intravenous injection (0.5 mg/kg) 30 minutes before exercise. Horses performed submaximal exercise (6 m/s for 5 minutes) followed by maximal exercise (until fatigue). Arterial blood samples were collected before exercise, during exercise, and immediately post-exercise to measure oxygen-related parameters, heart rate, and blood lactate [1] - Horse and camel pharmacokinetic experiment: Healthy adult horses (400-450 kg) and camels (450-500 kg) were administered Tripelennamine HCl (1 mg/kg) via intravenous injection. Blood samples were collected at 0, 5, 15, 30 minutes, 1, 2, 4, 8, 12, 24 hours post-administration. Plasma drug concentration was measured via high-performance liquid chromatography (HPLC) to calculate pharmacokinetic parameters [2] |
| 药代性质 (ADME/PK) |
Humans: Oral bioavailability is 60-65%; peak plasma concentration (Cmax) is reached at 1-2 hours post-oral administration (50 mg dose: Cmax=190 ng/mL) [3]
- Humans: Volume of distribution (Vd) is 2.8 L/kg; distributes widely into tissues [3] - Humans: Metabolized in the liver via cytochrome P450 (CYP) 2D6 and 3A4; primary metabolite is inactive [3] - Humans: 70% of the dose is excreted in urine (40% as unchanged drug, 30% as metabolites), 25% in feces. Elimination half-life (t1/2) is 6-8 hours [3] - Humans: Plasma protein binding rate is 85-90% [3] - Horses: After intravenous administration (1 mg/kg), Vd=1.5 L/kg, t1/2=4.2 hours, total body clearance (Cl)=0.26 L/kg/h [2] - Camels: After intravenous administration (1 mg/kg), Vd=1.8 L/kg, t1/2=5.8 hours, Cl=0.21 L/kg/h [2] |
| 参考文献 |
|
| 其他信息 |
Tripelenamine hydrochloride appears as odorless white crystalline powder or solid. Bitter taste. Solutions are neutral to litmus. pH of aqueous solution (25 mg/mL): 6.71. pH of aqueous solution (50 mg/mL): 6.67. pH of aqueous solution (100 mg/mL): 5.56. (NTP, 1992)
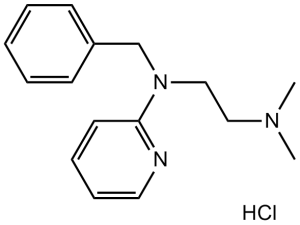
Tripelennamine Hydrochloride is the hydrochloride salt form of tripelennamine, an ethylenediamine derivative with an antihistaminergic property. Tripelennamine hydrochloride competitively blocks central and peripheral histamine H1 receptors, thereby limiting histamine's effects, including bronchoconstriction, vasodilation, increased capillary permeability, and spasmodic contractions of gastrointestinal smooth muscle. In addition, this agent binds to muscarinic receptors, resulting in anticholinergic activity. A histamine H1 antagonist with low sedative action but frequent gastrointestinal irritation. It is used to treat ASTHMA; HAY FEVER; URTICARIA; and RHINITIS; and also in veterinary applications. Tripelennamine is administered by various routes, including topically. See also: Tripelennamine (has active moiety). Tripelennamine HCl is a first-generation histamine H1 receptor antagonist [1] Its core mechanism is competitive antagonism of H1R to block histamine-mediated allergic responses [1] Indications include allergic rhinitis, urticaria, and pruritus, relieving symptoms such as sneezing and skin itching [1] It does not affect arterial hypoxemia or exercise performance in Thoroughbred horses at the tested dose (0.5 mg/kg, intravenous) [1] Pharmacokinetic parameters vary across species: camels have a longer elimination half-life than horses, while humans show higher volume of distribution than both species [2,3] Oral administration in humans results in moderate bioavailability, with primary excretion via urine [3] |
| 分子式 |
C16H22CLN3
|
|
|---|---|---|
| 分子量 |
291.82
|
|
| 精确质量 |
291.15
|
|
| 元素分析 |
C, 65.85; H, 7.60; Cl, 12.15; N, 14.40
|
|
| CAS号 |
154-69-8
|
|
| 相关CAS号 |
Tripelennamine; 91-81-6; Tripelennamine citrate; 6138-56-3
|
|
| PubChem CID |
9066
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.20
|
|
| 沸点 |
387.8ºC at 760 mmHg
|
|
| 熔点 |
192-193ºC
|
|
| 闪点 |
188.3ºC
|
|
| 蒸汽压 |
3.21E-06mmHg at 25°C
|
|
| LogP |
3.451
|
|
| tPSA |
19.37
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
6
|
|
| 重原子数目 |
20
|
|
| 分子复杂度/Complexity |
236
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
Cl[H].N(C1=C([H])C([H])=C([H])C([H])=N1)(C([H])([H])C1C([H])=C([H])C([H])=C([H])C=1[H])C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])[H]
|
|
| InChi Key |
FSSICIQKZGUEAE-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C16H21N3.ClH/c1-18(2)12-13-19(16-10-6-7-11-17-16)14-15-8-4-3-5-9-15;/h3-11H,12-14H2,1-2H3;1H
|
|
| 化学名 |
N'-benzyl-N,N-dimethyl-N'-pyridin-2-ylethane-1,2-diamine;hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.57 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.57 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (8.57 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 33.33 mg/mL (114.21 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4268 mL | 17.1338 mL | 34.2677 mL | |
| 5 mM | 0.6854 mL | 3.4268 mL | 6.8535 mL | |
| 10 mM | 0.3427 mL | 1.7134 mL | 3.4268 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|
|