| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
HDAC6 ( IC50 = 15 nM ); HDAC8 ( IC50 = 854 nM ); HDAC1 ( IC50 = 16400 nM )
|
|
|---|---|---|
| 体外研究 (In Vitro) |
体外活性:Tubastatin A 对所有 11 种 HDAC 异构体均具有显着选择性,并且对除 HDAC8 之外的所有异构体保持超过 1000 倍的选择性,其中 HDAC8 的选择性约为 57 倍。在同型半胱氨酸 (HCA) 诱导的神经变性测定中,Tubastatin A 从 5 μM 开始,对 HCA 诱导的神经元细胞死亡表现出剂量依赖性保护作用,在 10 μM 时几乎完全保护。 100 ng/mL 的 Tubastatin A 可增强 Foxp3+ T 调节细胞 (Treg) 对体外 T 细胞增殖的抑制作用。当 α-微管蛋白在生肌过程早期过度乙酰化时,C2C12 细胞中的图巴他汀 A 治疗会导致肌管形成受损。然而,当肌管中的α-微管蛋白过度乙酰化时,就会发生肌管伸长。最近的一项研究表明,原子力显微镜 (AFM) 测试表明,图巴他汀 A 治疗可增加细胞弹性,且不会对小鼠卵巢癌细胞系 MOSE-E 和 MOSE-L 中的肌动蛋白微丝或微管网络产生剧烈变化。激酶测定:酶抑制测定由位于宾夕法尼亚州 Malvern 的 Reaction Biology Corporation 使用 Reaction Biology HDAC Spectrum 平台进行。 (www.reactionbiology.com) HDAC1、2、4、5、6、7、8、9、10 和 11 检测使用分离的重组人蛋白; HDAC3/NcoR2 复合物用于 HDAC3 测定。 HDAC1、2、3、6、10 和 11 测定的底物是来自 p53 残基 379-382 的荧光肽 (RHKKAc); HDAC8 的底物是基于 p53 (RHKAcKAc) 残基 379-382 的荧光二酰肽。乙酰基-Lys(三氟乙酰基)-AMC 底物用于 HDAC4、5、7 和 9 测定。将图巴他汀 A 溶解在 DMSO 中,并以 10 剂量 IC50 模式进行测试,从 30 μM 开始进行 3 倍系列稀释。对照化合物曲古抑菌素 A (TSA) 在 10 剂量 IC50 中进行测试,从 5 μM 开始进行 3 倍连续稀释。 IC50 值通过曲线拟合剂量/反应斜率来提取。细胞测定:如前所述,从胎儿 Sprague-Dawley 大鼠(胚胎第 17 天)的大脑皮层获得原代皮层神经元培养物。所有实验均在电镀后 24 小时开始。在这些条件下,细胞不易受到谷氨酸介导的兴奋性毒性的影响。对于细胞毒性研究,用温 PBS 冲洗细胞,然后置于含有 5.5 g/L 葡萄糖、10% 胎牛血清、2 mM L-谷氨酰胺和 100 μM 胱氨酸的最低必需培养基 (Invitrogen) 中。通过向培养基中添加谷氨酸类似物同型半胱氨酸(HCA;5 mM)来诱导氧化应激。 HCA 由 100 倍浓缩溶液稀释而成,并调节至 pH 7.5。结合 HCA,用指定浓度的图巴他汀 A 处理神经元。 24小时后通过MTT测定(3-(4,5-二甲基噻唑-2-基)-2,5-二苯基四唑溴化物)方法评估活力。
|
|
| 体内研究 (In Vivo) |
每日治疗 0.5mg/kg 的 Tubastatin A 可抑制 HDAC6,从而促进炎症和自身免疫小鼠模型中的 Tregs 抑制活性,包括多种形式的实验性结肠炎和完全主要组织相容性复合体 (MHC) 不相容的心脏同种异体移植排斥反应。
|
|
| 酶活实验 |
反应生物学 HDAC Spectrum 平台用于执行酶抑制实验。分离的重组人蛋白用于 HDAC1、2、4、5、6、7、8、9、10 和 11 测定; HDAC3/NcoR2 复合物用于 HDAC3 测试。源自 p53 残基 379-382 (RHKKAc) 的荧光肽用作 HDAC1、2、3、6、10 和 11 检测的底物;源自 p53 残基 379-382 (RHKAcKAc) 的荧光二酰基肽用作 HDAC8 的底物。对于 HDAC4、5、7 和 9 测定,使用乙酰基-Lys(三氟乙酰基)-AMC 底物。将图巴他汀 A 溶解在 DMSO 中后,使用从 30 μM 开始的 3 倍连续稀释方案在 10 剂量 IC50 模式下进行测试。使用从 5 μM 开始的 3 倍连续稀释,在 10 剂量 IC50 中测试对照化合物曲古抑菌素 A (TSA)。对剂量/反应斜率进行曲线拟合即可得出 IC50 值。
|
|
| 细胞实验 |
胎儿 Sprague-Dawley 大鼠(胚胎第 17 天)的大脑皮层用于培养初级皮层神经元。电镀后二十四小时,开始所有实验。在这些情况下,谷氨酸介导的兴奋性毒性不会损害细胞。将细胞用温 PBS 洗涤,然后放入含有 5.5 g/L 葡萄糖、10% 胎牛血清、2 mM L-谷氨酰胺和 100 μM 胱氨酸的基本必需培养基中进行细胞毒性研究。将谷氨酸类似物同型半胱氨酸 (HCA;5 mM) 添加到培养基中以引起氧化应激。 HCA是通过稀释已浓缩100倍并调节pH至7.5的溶液来制备的。除了 HCA 之外,还用指定浓度的图巴他汀 A 处理神经元。 MTT 测定(3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化四唑)用于测定 24 小时后的活力。
|
|
| 动物实验 |
|
|
| 参考文献 |
|
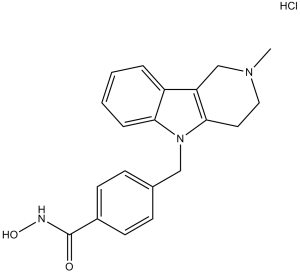
| 分子式 |
C20H21N3O2.HCL
|
|---|---|
| 分子量 |
371.86
|
| 精确质量 |
371.14
|
| 元素分析 |
C, 64.60; H, 5.96; Cl, 9.53; N, 11.30; O, 8.60
|
| CAS号 |
1310693-92-5
|
| 相关CAS号 |
1310693-92-5 (HCl); 1252003-15-8
|
| 外观&性状 |
White to yellow solid powder
|
| LogP |
3.9273
|
| tPSA |
57.5Ų
|
| SMILES |
CN1CCC2=C(C1)C3=CC=CC=C3N2CC4=CC=C(C=C4)C(=O)NO.Cl
|
| InChi Key |
LJTSJTWIMOGKRJ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C20H21N3O2.ClH/c1-22-11-10-19-17(13-22)16-4-2-3-5-18(16)23(19)12-14-6-8-15(9-7-14)20(24)21-25;/h2-9,25H,10-13H2,1H3,(H,21,24);1H
|
| 化学名 |
N-hydroxy-4-[(2-methyl-3,4-dihydro-1H-pyrido[4,3-b]indol-5-yl)methyl]benzamide;hydrochloride
|
| 别名 |
AG-CR-13900; TubA; Tubastatin A hydrochloride; Tubastatin A HCl; TSA HCl
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.62 mg/mL (7.05 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.62 mg/mL (7.05 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (5.59 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.08 mg/mL (5.59 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100μL 20.8mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 5 中的溶解度: ≥ 2.08 mg/mL (5.59 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 配方 6 中的溶解度: ≥ 0.52 mg/mL (1.40 mM) (饱和度未知) in 1% DMSO 99% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 7 中的溶解度: 1% DMSO+30% polyethylene glycol+1% Tween 80: 30 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6892 mL | 13.4459 mL | 26.8918 mL | |
| 5 mM | 0.5378 mL | 2.6892 mL | 5.3784 mL | |
| 10 mM | 0.2689 mL | 1.3446 mL | 2.6892 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。