| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |
| 靶点 |
ERK1; ERK2 (IC50 <0.3 nM)
|
|---|---|
| 体外研究 (In Vitro) |
Ulixertinib 降低具有 b-RAFV600E 突变的 A375 黑色素瘤细胞系中磷酸化的 ERK2 (pERK) 和下游激酶 RSK (pRSK) 的磷酸化,IC50 值分别为 4.1/0.14 μM。 ulixertinib 的 IC50 为 180 nM,还可以防止 A375 细胞增殖。 [1]
Ulixertinib(BVD-523,VRT752271)是一种新型小分子,以可逆的ATP竞争方式有效和选择性地抑制ERK1和ERK2激酶[2]。 |
| 体内研究 (In Vivo) |
在药代动力学研究中,发现该测定的敏感性和特异性足以准确表征 Ulixertinib (VRT752271) 在 Balb/C 小鼠中的血浆药代动力学。
Ulixertinib在BRAF突变黑色素瘤和结直肠异种移植物以及KRAS突变结直肠和胰腺模型中抑制体内肿瘤生长。在临床研究中,晚期实体瘤患者对ulixertinib具有良好的耐受性。在一项口服I期剂量递增研究(共9剂)中,以确定剂量限制毒性(DLT)、最大耐受剂量(MTD)以及药代动力学特征和初步疗效评估为终点,以10-900mg的剂量范围以b.I.d方案给药。Ulixertinib显示出高达600mg(b.i.d)的线性药代动力学,这被发现是MTD。[2] 已开发并验证了一种灵敏、特异和快速的LC-ESI-MS/MS方法,用于根据监管指南使用非那西丁作为内标(I.S.)定量小鼠血浆中的ulixertinib。样品制备是通过用乙腈:甲醇混合物进行蛋白质沉淀程序完成的。在Atlantis dC18柱上使用二元梯度进行色谱分离,流动相为a(0.2%甲酸水溶液)和B(乙腈),流速为0.60mL/min。ulixertinib和I.S.的洗脱分别发生在1.07和1.20min。色谱总运行时间为2.5min。在1.58-2054ng/mL的浓度范围内建立了线性响应函数。日内和日间准确度和精密度分别在2.11-11.8%和5.80-11.4%的范围内。这种新方法已应用于小鼠的药代动力学研究[2]。 |
| 酶活实验 |
MEK U911 激活的 ERK2 蛋白在内部表达和纯化。酶和底物溶液在测定缓冲液中制备,该缓冲液由 50 mM Tris (pH 7.5)、10 mM MgCl2、0.1 mM EGTA、10 mM DTT 和 0.01% (v/v) CHAPS 组成。装有测试和参考对照物质的聚丙烯 384 孔板中装有 10 µL 在测定缓冲液中制备的 1.2 nM ERK2 蛋白。为了确定化合物的 IC50,预先对化合物板进行了从 100 µM 到 0.1 nM 的 12 点范围的剂量,测定中的总 DMSO 浓度为 1%。在室温下预孵育 20 分钟后,添加 10 µL 底物溶液(由测定缓冲液中的 16 µM erktide (IPTTPITTTYFFFK) 和 120 µM ATP(测量的 Km)组成)。在室温下反应 20 分钟后,添加 80 µl 1% (v/v) 甲酸淬灭反应。然后使用 RapidFire 质谱平台运行测定板,以测量底物(未磷酸化 Erktide)和产物(磷酸化 Erktide)水平。
|
| 细胞实验 |
A375 细胞在由 DMEM、10% (v/v) 胎牛血清和 1% (v/v) L-谷氨酰胺组成的细胞培养基中培养。收获细胞,分配到黑色 384 孔 Costar 板中,每孔 200 个细胞,细胞培养基总体积为 40 L,然后在旋转培养箱中在 37°C、90% 相对湿度和 5% CO2 下培养过夜。使用 Labcyte Echo 555 声学分配器,将测试物质和参考对照直接添加到细胞板内部 308 个孔中。测定中 DMSO 总浓度为 0.3%,在从 30 M 到 0.03 nM 的 12 个点范围内对细胞进行给药,以确定化合物的 IC50。随后,将细胞板在 37°C 下孵育 72 小时。为了固定和染色细胞,将 20 µL 12% 甲醛与 1:2000 稀释的 Hoechst 33342 一起添加到 PBS/A(4% 最终浓度)中。然后将细胞在室温下孵育 30 分钟,然后用 PBS/A 洗涤。 Cellomics ArrayScanTM VTI 成像平台用于对染色细胞板上的细胞进行计数。此外,对第 0 天的细胞板进行固定、染色和读取,以产生基线细胞计数,用于评估化合物的抗增殖和细胞毒性作用。
|
| 动物实验 |
Pharmacokinetic study [2]
Male Balb/C mice (n = 24) were housed in Jubilant Biosys animal house facility at 22 ± 2 °C and at humidity (30–70%) controlled room (15 air changes/h) with a 12:12 h light:dark cycles, had free access to rodent feed and water for one week before using for experimental purpose. Following ∼4 h fast (during the fasting period animals had free access to water) animals were divided into two groups (n = 12/group). Group I animals (25–28 g) received Ulixertinib orally at 10 mg/kg (strength: 1.0 mg/mL; dose volume: 10 mL/kg), whereas Group II animals (29–31 g) received Ulixertinib intravenously (strength: 0.1 mg/mL; dose volume: 10 mL/kg) at 1.0 mg/kg dose. Post-dosing serial blood samples (50 μL, sparse sampling was done and at each time point three mice were used for blood sampling) were collected using Micropipettes through tail vein into polypropylene tubes containing Na2·EDTA solution as an anti-coagulant at 0.25, 0.5, 1, 2, 4, 8, 10 and 24 (for oral study) and 0.12, 0.25, 0.5, 1, 2, 4, 8 and 24 (for intravenous study). Plasma was harvested by centrifuging the blood using Biofuge at 1760 g for 5 min and stored frozen at −80 ± 10 °C until analysis. Animals were allowed to access feed 2 h post-dosing. The criteria for acceptance of the analytical runs encompassed the following: (i) 67% of the QC samples accuracy must be within 85–115% of the nominal concentration (ii) not less than 50% at each QC concentration level must meet the acceptance criteria. Plasma concentration-time data of Ulixertinib was analyzed by non-compartmental method using Phoenix WinNonlin Version 6.3. |
| 参考文献 |
|
| 其他信息 |
Ulixertinib is a a novel, reversible, ATP-competitive ERK1/2 inhibitor with high potency and ERK1/2 selectivity. It is currently in clinical trials for the treatment of a wide range of tumors.
Ulixertinib is an orally available inhibitor of extracellular signal-regulated kinase (ERK) 1 and 2, with potential antineoplastic activity. Upon oral administration, ulixertinib inhibits both ERK 1 and 2, thereby preventing the activation of ERK-mediated signal transduction pathways. This results in the inhibition of ERK-dependent tumor cell proliferation and survival. The mitogen-activated protein kinase (MAPK)/ERK pathway is often upregulated in a variety of tumor cell types and plays a key role in tumor cell proliferation, differentiation and survival. The RAS/RAF/MEK/ERK signaling pathway has been targeted with a number of small molecule inhibitors in oncology clinical development across multiple disease indications. Importantly, cell lines with acquired resistance to B-RAF and MEK inhibitors have been shown to maintain sensitivity to ERK1/2 inhibition by small molecule inhibitors. There are a number of selective, noncovalent ERK1/2 inhibitors reported along with the promiscuous hypothemycin (and related analogues) that act via a covalent mechanism of action. This article reports the identification of multiple series of highly selective covalent ERK1/2 inhibitors informed by structure-based drug design (SBDD). As a starting point for these covalent inhibitors, reported ERK1/2 inhibitors and a chemical series identified via high-throughput screening were exploited. These approaches resulted in the identification of selective covalent tool compounds for potential in vitro and in vivo studies to assess the risks and or benefits of targeting this pathway through such a mechanism of action. [1] Pancreatic ductal adenocarcinoma (PDAC) is a deadly disease in urgent need of newer therapeutic modalities. Majority of patients with PDAC have mutations in KRAS, which unfortunately remains an ineffectual target. Our strategy here is to target KRAS downstream effectors PI3K and mTOR. In this study, we investigated the antitumor efficacy of the novel PI3K and mTOR dual inhibitor VS-5584 in PDAC. Our data shows that PI3K/mTOR dual inhibition causes ERK activation in all tested PDAC cell lines. Although the MEK inhibitor GSK1120212 could abrogate VS-5584-induced ERK activation, it did not substantially enhance cell death in all the cell lines tested. However, combination with ERK inhibitor SCH772984 not only mitigated VS-5584-induced ERK activation but also enhanced VS-5584-induced cell death. In a xenograft model of PDAC, we observed 28% and 44% tumor inhibition for individual treatment with VS-5584 and SCH772984, respectively, while the combined treatment showed superior tumor inhibition (80%) compared to vehicle control treatment. Our findings support the clinical development of VS-5584 and ERK inhibitor combination for PDAC treatment. [3] |
| 分子式 |
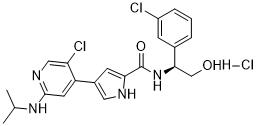
C21H23CL3N4O2
|
|---|---|
| 分子量 |
469.792
|
| 精确质量 |
468.088
|
| 元素分析 |
C, 53.69; H, 4.93; Cl, 22.64; N, 11.93; O, 6.81
|
| CAS号 |
1956366-10-1
|
| 相关CAS号 |
Ulixertinib;869886-67-9
|
| PubChem CID |
71584481
|
| 外观&性状 |
White to off-white solid powder
|
| tPSA |
90
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
539
|
| 定义原子立体中心数目 |
1
|
| SMILES |
CC(C)NC1=NC=C(C(=C1)C2=CNC(=C2)C(=O)N[C@H](CO)C3=CC(=CC=C3)Cl)Cl.Cl
|
| InChi Key |
DKGYQCPFBWFTHM-FSRHSHDFSA-N
|
| InChi Code |
InChI=1S/C21H22Cl2N4O2.ClH/c1-12(2)26-20-8-16(17(23)10-25-20)14-7-18(24-9-14)21(29)27-19(11-28)13-4-3-5-15(22)6-13;/h3-10,12,19,24,28H,11H2,1-2H3,(H,25,26)(H,27,29);1H/t19-;/m1./s1
|
| 化学名 |
N-[(1S)-1-(3-chlorophenyl)-2-hydroxyethyl]-4-[5-chloro-2-(propan-2-ylamino)pyridin-4-yl]-1H-pyrrole-2-carboxamide;hydrochloride
|
| 别名 |
BVD-523; VRT752271BVD-523; Ulixertinib hydrochloride; 1956366-10-1; Ulixertinib HCl; Ulixertinib (hydrochloride); 3K792HRQ8B; BVD 523; BVD523; VRT752271; VRT752271; VRT 752271; Ulixertinib
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 86~100 mg/mL (198.5~212.9 mM)
Ethanol: ~86 mg/mL (~198.5 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.43 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.43 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.43 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 5%DMSO+40%PEG300+5%Tween80+50%ddH2O: 4.3mg/ml 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1286 mL | 10.6431 mL | 21.2861 mL | |
| 5 mM | 0.4257 mL | 2.1286 mL | 4.2572 mL | |
| 10 mM | 0.2129 mL | 1.0643 mL | 2.1286 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02608229 | Terminated | Drug: BVD-523 Drug: Gemcitabine |
Pancreatic Cancer Pancreas Cancer |
Washington University School of Medicine |
June 6, 2016 | Phase 1 |