| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
FLT3 (IC50 = 0.35 nM); Mer (IC50 = 0.46 nM); Axl (IC50 = 1.65 nM); TrkA (IC50 = 1.67 nM); TrkC (IC50 = 4.38 nM)
UNC-2025 targets MERTK (Ki = 0.15 nM) [1] UNC-2025 targets FLT3 (IC50 = 1.8 nM); exhibits selectivity over other kinases: c-KIT (IC50 = 45 nM), RET (IC50 = 62 nM), EGFR (IC50 > 1000 nM), VEGFR2 (IC50 > 1000 nM) [1] |
|---|---|
| 体外研究 (In Vitro) |
体外活性:UNC-2025是一种新型、有效、口服生物可利用的MER/FLT3双重抑制剂,IC50分别为0.74 nM和0.8 nM,其选择性是Axl和Tyro3的约20倍。 UNC-2025 能够抑制体内 Mer 磷酸化。体外对 300 多种激酶的激酶组分析和细胞选择性评估表明,UNC-2025 对急性髓性白血病 (AML) 的另一个重要靶点 Flt3 具有相似的亚纳摩尔活性,与其他检查的激酶相比具有药理学上有用的选择性。激酶测定:UNC2025 盐酸盐是一种有效的口服生物可利用的 Mer/Flt3 双重抑制剂,对 Mer/Flt3 的 IC50 为 0.8/0.74 nM。口服给药后,UNC2025 能够在体内抑制 Mer 磷酸化,这一点通过检查小鼠骨髓白血病细胞中磷酸化 Mer 的药效 (PD) 研究证明。体外对 300 多种激酶进行的激酶组分析和细胞选择性评估表明,11 种激酶对急性髓性白血病 (AML) 的另一个重要靶点 Flt3 具有相似的亚纳摩尔活性,与其他检查的激酶相比具有药理学上有用的选择性。细胞测定:在 697 B-ALL 细胞中,UNC-2025 有效抑制 Mer 磷酸化,IC50 为 2.7 nM。在 A549 NSCLC 和 Molm-14 AML 细胞系中,UNC-2025 显着抑制依赖于 Mer8 和 Flt3 的集落形成。在 H2228 和 H1299 细胞系中,UNC-2025 抑制下游 MERTK 致癌信号传导,例如基础和刺激的 pAKT 和 pERK1/2。在四种 NSCLC 细胞系中,UNC-2025 还诱导细胞凋亡,并减少集落形成。
重组激酶活性实验显示,UNC-2025 强效抑制MERTK和FLT3,对c-KIT和RET的选择性>300倍,对EGFR/VEGFR2的选择性>500倍[1] - 在FLT3-ITD阳性白血病细胞系(MV4-11、MOLM-13)中,UNC-2025(0.01–100 nM)以剂量依赖性方式抑制细胞增殖(MV4-11的IC50 = 3.2 nM;MOLM-13的IC50 = 4.5 nM),并诱导凋亡(10 nM时MV4-11的Annexin V-FITC/PI染色显示凋亡率约55%)[2] - 它阻断MERTK/FLT3下游信号通路:Western blot检测显示MV4-11细胞中MERTK(Tyr749)、FLT3(Tyr591)、AKT(Ser473)、ERK1/2(Thr202/Tyr204)和STAT5(Tyr694)的磷酸化水平降低,不影响总蛋白水平[2] - 与BCL-2抑制剂CL14377联合使用时,UNC-2025(1 nM)协同抑制MV4-11细胞增殖(联合指数=0.45),凋亡率提升至约75%(单药组为30%)[2] - 在MERTK过表达白血病细胞(K562-MERTK)中,UNC-2025(0.1–10 nM)抑制细胞增殖(IC50 = 2.8 nM),并减少MERTK介导的凋亡细胞吞噬(5 nM时减少约60%)[2] |
| 体内研究 (In Vivo) |
在携带 697 个急性白血病肿瘤的小鼠中,UNC-2025(3 mg/kg,口服)显示出良好的溶解度和 DMPK 特性,并产生有效的靶标抑制作用。在携带 H2228 或 A549 肿瘤的小鼠中,UNC-2025(50 mg/kg,口服)可抑制肿瘤生长。
UNC2025在异种移植物模型中具有显著的治疗效果,无论起始疾病负担如何,其肿瘤负担的剂量依赖性降低和中位生存期的两倍一致增加。在患者源性AML异种移植模型中,UNC2025治疗可诱导疾病消退。此外,UNC2025在体内增加了对甲氨蝶呤的敏感性,这表明在目前的细胞毒性方案中加入mertk靶向治疗可能特别有效和/或允许化疗剂量减少。结论:UNC2025在白血病患者样本和异种移植模型中介导的广谱活性,单独或联合细胞毒性化疗,支持了MERTK抑制剂治疗白血病的持续发展。[2] 在MV4-11(FLT3-ITD+)皮下异种移植模型(裸鼠)中:口服UNC-2025(25 mg/kg/天)持续21天,较溶媒对照组抑制肿瘤生长约78%。肿瘤组织中p-MERTK、p-FLT3、p-STAT5和Ki-67表达降低,切割型半胱天冬酶-3水平升高(免疫组织化学和Western blot检测)[2] - 在MOLM-13(FLT3-ITD+)静脉注射白血病模型(NSG小鼠)中:口服UNC-2025(25 mg/kg/天)持续28天,中位生存期从对照组的22天延长至46天。骨髓和脾脏中白血病细胞浸润减少(流式细胞术:CD45+CD33+细胞减少约65%)[2] - 联合治疗:在MV4-11异种移植模型中,口服UNC-2025(15 mg/kg/天)+ 口服CL14377(50 mg/kg/天)持续21天,肿瘤生长抑制率达约90%,中位生存期延长至72天,且无毒性增加[2] |
| 酶活实验 |
ActivX ATP/ADP探针的Kinome分析[1]
简单地说,将697个B-ALL细胞轻轻成粒,用PBS洗涤两次,用MPER添加HALT蛋白酶/磷酸酶抑制剂鸡尾酒进行裂解,并用Zeba凝胶过滤自旋柱去除残留的ATP和ADP。过滤后,使用反应缓冲液调整最终蛋白浓度至5.0 mg/mL,并添加1X HALT蛋白酶和磷酸酶抑制剂混合物。裂解液被引用,在液氮中快速冷冻,并在- 80°C保存直到标记。标记前,将总裂解物2.5 mg(终体积500 μL)解冻至室温,用10 μL 1 M MnCl2处理1 min,然后用或不加UNC2025[0、0.01、0.1、1.0、10、100和1000 nM]处理10 min。处理后,以终浓度5 μM加入ATP探针10 min。标记反应用500 μL 10 M尿素在MPER中,10 μL 500 mM DTT淬火,加热至65℃,摇晃30 min。将样品冷却至室温,用40 μL的1 M碘乙酰胺溶液避光烷基化30 min。经Zeba凝胶过滤,用20 μg胰蛋白酶在37℃下振荡消化2 h。加入50 μL的50%高容量链亲和素琼脂糖浆液,室温下在旋转器上恒定混合孵育1 h。然后捕获琼脂糖珠,洗涤和洗脱。纯化肽冷冻、冻干,保存于- 80°C。在质谱分析之前,肽在25 μL 0.1% TFA中重悬。质谱分析和数据分析的详细信息在辅助信息中提供。 基于细胞的激酶抑制试验[1] 697 B-ALL细胞和Molm-14 AML细胞在UNC2025存在下或仅在培养液中培养1.0 h。将20 mM正钒酸钠与0.3% (w/w)过氧化氢在0.9× PBS中按1:1的比例混合,在室温下制备新鲜的过钒酸盐溶液15-20 min。培养物在收集前用120 μM的过氧化物酸盐处理3分钟,细胞裂解液在50 mM HEPES (pH 7.5)、150 mM NaCl、10 mM EDTA、10%甘油和1% Triton X-100中制备,并添加蛋白酶抑制剂。用抗Mer或抗Flt3抗体和蛋白G琼脂糖珠免疫沉淀Mer和Flt3蛋白。磷酸化蛋白通过Western blot检测,使用针对Mer8三磷酸化激活环衍生的肽或磷酸化Flt3特异性抗体的抗磷酸化mer抗体。剥离硝化纤维素膜,用第二抗mer抗体或抗flt3抗体检测总蛋白。通过ImageJ密度测定相对磷酸化蛋白和总蛋白水平,并通过非线性回归计算IC50值。 UNC2025盐酸对Mer/Flt3的IC50为0.8/0.74 nM,是一种强效的口服Mer/Flt3双抑制剂。使用药效学(PD)方法观察小鼠骨髓白血病母细胞磷酸化-Mer的研究表明,口服给药后,UNC2025可以抑制体内磷酸化。对300多种激酶的体外激酶组分析和细胞选择性评估结果表明,与其他检测的激酶相比,UNC2025具有药理学上有用的选择性,并且对Flt3具有相似的亚纳微活性,Flt3是急性髓性白血病(AML)的另一个重要靶点。 MERTK激酶活性实验:重组人MERTK(10 nM)与多聚(Glu-Tyr)底物、ATP和反应缓冲液(20 mM Tris-HCl pH 7.5、10 mM MgCl2、1 mM DTT)在30°C孵育60分钟。加入浓度范围为0.001–10 nM的UNC-2025,使用[γ-32P]ATP通过放射测量法检测磷酸化底物。Lineweaver-Burk图分析计算Ki值[1] - FLT3激酶活性实验:重组人FLT3(20 nM)与FLT3衍生肽底物、ATP和反应缓冲液在30°C孵育45分钟。加入UNC-2025(0.01–100 nM),HTRF法(激发光340 nm,发射光665 nm)检测磷酸化肽段。剂量-反应曲线非线性回归确定IC50值[1] - 激酶选择性面板实验:UNC-2025(100 nM)与45种纯化人激酶(包括c-KIT、RET、EGFR、VEGFR2)及相应底物/ATP在标准条件下孵育。放射测量法或荧光法检测激酶活性,计算抑制百分比以评估选择性[1] |
| 细胞实验 |
软琼脂菌落形成试验[1]
A549或Molm-14细胞在1.5 mL含有1倍RPMI培养基和10% FBS的0.35%软琼脂中培养,并覆盖2.0 mL含有10% FBS的1倍RPMI培养基和指示浓度的UNC2025或DMSO载体。中、<强>UNC2025或车辆每周刷新3次。用硝基四氮唑蓝染色,2周后计数。 免疫印迹分析[1] 白血病细胞(3x106/mL)用UNC2025或相当于300nM UNC2025的DMSO培养1小时。制备细胞裂解液,免疫印迹法检测信号蛋白。细胞用过钒酸盐处理,免疫沉淀MERTK检测磷酸化的MERTK。 细胞凋亡、细胞周期和集落形成的研究[1] 细胞用UNC2025或DMSO (3 × 10~5/mL)培养6、24和/或48小时。采用yo - pro -1碘化染色和碘化丙啶染色,流式细胞术检测凋亡细胞和死亡细胞;采用流式细胞术评估碘化丙啶染色,测定细胞周期谱;以MTT减少率作为活细胞数指标。或者,治疗后,ALL细胞系和患者样本在甲基纤维素中培养。AML细胞系在0.35% Noble琼脂中培养,覆盖含有UNC2025的培养基或载体。用含有UNC2025或DMSO的甲基纤维素培养正常骨髓或脐带血的人单核细胞。7天后(正常骨髓)或14天后(脐带血、细胞系和患者样本)计数菌落。 UNC-2025的IC50为2.7 nM,能有效抑制697 B-ALL细胞的Mer磷酸化。UNC-2025依赖于Flt3和Mer8,在A549 NSCLC和Molm-14 AML细胞系中显著抑制集落形成。UNC2025阻断H2228和H1299细胞系下游MERTK致癌信号,包括基础和刺激pAKT和pERK1/2。此外,在四种非小细胞肺癌细胞系中,UNC-2025抑制集落形成并引发凋亡细胞死亡。 白血病细胞增殖及凋亡实验:MV4-11/MOLM-13/K562-MERTK细胞(每孔5×10³个)接种于96孔板,用UNC-2025(0.01–100 nM)处理72小时。CCK-8法检测细胞活力以确定IC50。凋亡实验中,细胞用药物(1–10 nM)处理48小时,Annexin V-FITC/PI染色后流式细胞仪分析[2] - 信号通路实验:MV4-11细胞(每孔1×10⁶个)接种于6孔板,血清饥饿16小时后,用UNC-2025(1–10 nM)处理24小时。细胞裂解后,Western blot检测p-MERTK、MERTK、p-FLT3、FLT3、p-AKT、AKT、p-ERK1/2、ERK1/2、p-STAT5、STAT5和GAPDH[2] - 联合治疗实验:MV4-11细胞用UNC-2025(0.1–10 nM)+ CL14377(10–100 nM)处理72小时。CCK-8法检测细胞活力,Chou-Talalay法计算联合指数[2] - 吞噬实验:K562-MERTK细胞与荧光标记的凋亡Jurkat细胞(MOI = 5)及UNC-2025(0.1–10 nM)孵育4小时。流式细胞仪量化吞噬率(阳性凋亡细胞荧光的K562-MERTK细胞)[2] |
| 动物实验 |
NOD/SCID/gamma mice
3 mg/kg Oral gavage Pharmacodynamic Studies[1] NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice were transplanted with 2 × 106 697 B-ALL cells by intravenous injection into the tail vein, and leukemia was established for 14 days prior to treatment with a single dose of 3 mg/kg 11 (UNC2025) or an equivalent volume (10 mL/kg) of saline vehicle. Pervanadate solution was prepared fresh, as described above. Femurs were collected from mice 30 min after treatment, and bone marrow cells were flushed with 1 mL of room temperature RPMI medium + 20% FBS + 1 μM MgCl2 + 100 untis/ml DNase + 240 μM pervanadate and incubated at room temperature in the dark for 10 min. Bone marrow cells were collected by centrifugation at 4 °C, lysates were prepared, Mer protein was immunoprecipitated, and total and phospho-Mer proteins were detected and quantitated by Western blot, as described above. Leukemia xenograft models[2] 697 cells, monoclonal 697 cells expressing firefly luciferase (20), NOMO-1 cells, or mononuclear cells from an AML patient sample (2x106/mouse) were injected into the tail vein in NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) or NOD.Cg-PrkdcscidIl2rgtm1WjlTg(CMV-IL3,CSF2,KITLG)1Eav/MloySzJ (NSGS) mice. Disease burden was monitored in 697-luciferase xenografts using bioluminescence imaging. Peripheral blood, spleen, and bone marrow were collected from patient-derived xenografts and red blood cells (RBCs) were lysed in 50% Dextran sulfate for 15 minutes. Human CD45+ cells were detected using flow cytometry. Mice were distributed to groups with statistically equal disease burden or randomized to groups if leukemia was undetectable. UNC2025 or saline was administered at 10ml/kg once daily by oral gavage. Methotrexate or saline was administered at 5ml/kg by intraperitoneal injection. Mice with advanced leukemia (>20% weight loss, tachypnea, hypothermia, hind-limb paralysis, minimal activity) were euthanized and survival was monitored. Pharmacodynamic studies were performed as previously described MV4-11 subcutaneous xenograft model: NSG mice (6-week-old, female) were subcutaneously injected with MV4-11 cells (5×10⁶ cells/mouse) into the right flank. When tumors reached ~100 mm³, mice were randomized into control (n = 6), UNC-2025 monotherapy (n = 6, 25 mg/kg/day, oral), and combination therapy (n = 6, UNC-2025 15 mg/kg/day + CL14377 50 mg/kg/day, oral) groups. Drugs were dissolved in 0.5% carboxymethylcellulose (CMC) + 0.1% Tween 80, administered once daily for 21 days. Tumor volume (length×width²/2) and body weight were measured every 3 days; tumors were excised for immunohistochemistry and Western blot [2] - MOLM-13 intravenous leukemia model: NSG mice (6-week-old, female) were intravenously injected with MOLM-13 cells (1×10⁶ cells/mouse). Seven days post-injection, mice were treated with UNC-2025 (25 mg/kg/day, oral) for 28 days. Survival time was recorded; bone marrow and spleen tissues were collected for flow cytometry analysis of leukemia cell infiltration [2] - Pharmacokinetic study: Male Sprague-Dawley rats (250–300 g) and beagle dogs (8–10 kg) were administered UNC-2025 via oral gavage (10 mg/kg) or intravenous injection (2 mg/kg). Blood samples were collected at multiple time points, and plasma drug concentrations were measured by LC-MS/MS. Pharmacokinetic parameters (Cmax, AUC, t1/2, F) were calculated using non-compartmental analysis [1] |
| 药代性质 (ADME/PK) |
As a result, analogue 11 (UNC2025) was prepared and demonstrated excellent PK properties: low clearance (9.2 mL/min kg), longer half-life (3.8 h), and high oral exposure (100%) (Table 2). Furthermore, the HCl salt of 11 was highly soluble in normal saline (kinetic solubility: 38 μg/mL, pH = 7.4). Substitution of 11 with a cyclopropyl ethyl side-chain resulted in 12, which demonstrated a further modest decrease in clearance, consistent with some contribution of P450 metabolism of the C3 side-chain to metabolic stability but with very similar overall PK properties to 11. With excellent solubility and PK properties, as well as a much less expensive C3 substituent versus 12, analogue 11 was chosen for further studies, including kinome selectivity profiling, cell-based assays, and pharmacodynamic assays using a mouse model to determine the activity of the compound in leukemic blasts in vivo.[1]
Oral bioavailability: 72% in rats, 68% in dogs [1] - Plasma half-life (t1/2): 4.1 hours in rats, 7.3 hours in dogs [1] - Plasma protein binding rate: 95% in human plasma, 93% in rat plasma, 94% in dog plasma (equilibrium dialysis assay) [1] - Tissue distribution: In rats, highest concentrations in liver (3.3-fold vs. plasma), spleen (2.9-fold vs. plasma), and bone marrow (2.5-fold vs. plasma); favorable penetration into hematopoietic tissues [1] - Metabolism: Primarily metabolized via hepatic CYP3A4-mediated oxidation; major metabolites are monohydroxylated derivatives (non-active) [1] - Excretion: In rats, 63% excreted in feces, 27% in urine within 72 hours post-administration [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
In vitro toxicity: UNC-2025 at concentrations up to 100 nM shows no significant cytotoxicity to normal human bone marrow mononuclear cells (BMNCs) (cell viability >85% vs. control) [2]
- Acute toxicity: LD50 > 2000 mg/kg in rats and mice (oral administration); no mortality or severe toxic symptoms (lethargy, convulsions) observed at doses up to 2000 mg/kg [1] - Repeat-dose toxicity: In a 28-day study in rats (oral doses of 10, 30, 60 mg/kg/day), the drug was well-tolerated. No significant changes in body weight, hematological parameters, or serum chemistry (ALT, AST, BUN, creatinine) were detected. Histological examination of liver, kidney, heart, and bone marrow revealed no abnormal lesions [1] - Combination therapy toxicity: Mice treated with UNC-2025 + CL14377 show no increased weight loss or organ toxicity compared to monotherapy groups [2] |
| 参考文献 | |
| 其他信息 |
We previously reported a potent small molecule Mer tyrosine kinase inhibitor UNC1062. However, its poor PK properties prevented further assessment in vivo. We report here the sequential modification of UNC1062 to address DMPK properties and yield a new potent and highly orally bioavailable Mer inhibitor, 11, capable of inhibiting Mer phosphorylation in vivo, following oral dosing as demonstrated by pharmaco-dynamic (PD) studies examining phospho-Mer in leukemic blasts from mouse bone marrow. Kinome profiling versus more than 300 kinases in vitro and cellular selectivity assessments demonstrate that 11 has similar subnanomolar activity against Flt3, an additional important target in acute myelogenous leukemia (AML), with pharmacologically useful selectivity versus other kinases examined.[1]
Purpose: MERTK tyrosine kinase is ectopically expressed in 30% to 50% of acute lymphoblastic leukemias (ALL) and more than 80% of acute myeloid leukemias (AML) and is a potential therapeutic target. Here, we evaluated the utility of UNC2025, a MERTK tyrosine kinase inhibitor, for treatment of acute leukemia.Experimental Design: Preclinical in vitro and in vivo assays using cell lines and primary leukemia patient samples were used to evaluate antileukemic effects of UNC2025.Results: UNC2025 potently inhibited prosurvival signaling, induced apoptosis, and reduced proliferation and colony formation in MERTK-expressing ALL and AML cell lines and patient samples. Approximately 30% of primary leukemia patient samples (78 of 261 total) were sensitive to UNC2025. Sensitive samples were most prevalent in the AML, T-ALL, and minimally differentiated (M0) AML subsets. UNC2025 inhibited MERTK in bone marrow leukemia cells and had significant therapeutic effects in xenograft models, with dose-dependent decreases in tumor burden and consistent two-fold increases in median survival, irrespective of starting disease burden. In a patient-derived AML xenograft model, treatment with UNC2025 induced disease regression. In addition, UNC2025 increased sensitivity to methotrexate in vivo, suggesting that addition of MERTK-targeted therapy to current cytotoxic regimens may be particularly effective and/or allow for chemotherapy dose reduction.Conclusions: The broad-spectrum activity mediated by UNC2025 in leukemia patient samples and xenograft models, alone or in combination with cytotoxic chemotherapy, supports continued development of MERTK inhibitors for treatment of leukemia.[2] UNC-2025 is a potent, orally bioavailable dual MERTK/FLT3 inhibitor [1] - Its mechanism of action involves binding to the ATP-binding pockets of MERTK and FLT3, inhibiting their kinase activity and downstream PI3K-AKT-ERK/STAT5 signaling pathways, leading to leukemia cell proliferation arrest and apoptosis [1,2] - It exhibits preclinical efficacy in FLT3-ITD-positive and MERTK-overexpressing leukemia models, both as monotherapy and in combination with BCL-2 inhibitors [2] - Favorable oral bioavailability, tissue distribution (especially to hematopoietic tissues), and low toxicity support its clinical application for hematological malignancies [1,2] - It has been evaluated in preclinical studies for acute myeloid leukemia (AML) and other leukemias with MERTK/FLT3 dysregulation [2] |
| 分子式 |
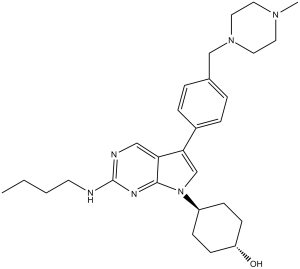
C28H40N6O
|
|---|---|
| 分子量 |
476.66
|
| 精确质量 |
476.326
|
| 元素分析 |
C, 70.55; H, 8.46; N, 17.63; O, 3.36
|
| CAS号 |
1429881-91-3
|
| 相关CAS号 |
UNC2025 hydrochloride;2070015-17-5
|
| PubChem CID |
73425588
|
| 外观&性状 |
White to light yellow solid
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
677.5±65.0 °C at 760 mmHg
|
| 闪点 |
363.5±34.3 °C
|
| 蒸汽压 |
0.0±2.2 mmHg at 25°C
|
| 折射率 |
1.655
|
| LogP |
3.09
|
| tPSA |
69.4
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
35
|
| 分子复杂度/Complexity |
627
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O([H])C1([H])C([H])([H])C([H])([H])C([H])(C([H])([H])C1([H])[H])N1C([H])=C(C2C([H])=C([H])C(=C([H])C=2[H])C([H])([H])N2C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C2([H])[H])C2=C([H])N=C(N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H])N=C12
|
| InChi Key |
MJSHVHLADKXCML-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C28H40N6O/c1-3-4-13-29-28-30-18-25-26(20-34(27(25)31-28)23-9-11-24(35)12-10-23)22-7-5-21(6-8-22)19-33-16-14-32(2)15-17-33/h5-8,18,20,23-24,35H,3-4,9-17,19H2,1-2H3,(H,29,30,31)
|
| 化学名 |
4-[2-(butylamino)-5-[4-[(4-methylpiperazin-1-yl)methyl]phenyl]pyrrolo[2,3-d]pyrimidin-7-yl]cyclohexan-1-ol
|
| 别名 |
UNC2025; UNC 2025; UNC-2025; mrx-6313; (1r,4r)-4-(2-(butylamino)-5-(4-((4-methylpiperazin-1-yl)methyl)phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)cyclohexanol; trans-4-(2-(Butylamino)-5-(4-((4-methylpiperazin-1-yl)methyl)phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)cyclohexanol; 4-[2-(butylamino)-5-[4-[(4-methylpiperazin-1-yl)methyl]phenyl]pyrrolo[2,3-d]pyrimidin-7-yl]cyclohexan-1-ol; CHEMBL3326006; UNC2025
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.24 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.24 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0979 mL | 10.4897 mL | 20.9793 mL | |
| 5 mM | 0.4196 mL | 2.0979 mL | 4.1959 mL | |
| 10 mM | 0.2098 mL | 1.0490 mL | 2.0979 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
UNC2025 inhibits colony formation in soft agar. Mol Cancer Ther. 2015 Sep;14(9):2014-22. |
UNC2025 Inhibits NSCLC tumor growth in vivo: H2228 (A) or A549 (B,C). Mol Cancer Ther. 2015 Sep; 14(9): 2014–2022. |
UNC2025 inhibits NSCLC tumor growth in vivo. Mol Cancer Ther. 2015 Sep;14(9):2014-22. |