| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
PARP-2 ( Ki = 2.9 nM ); PARP-1 ( Ki = 5.2 nM )
|
|---|---|
| 体外研究 (In Vitro) |
Veliparib 对 SIRT2 (>5 μM) 没有影响。 [1] veliparib 的 EC50 为 2 nM,可抑制 C41 细胞中的 PARP 活性[2]。在暴露和未暴露于光的 H460 细胞中,veliparib 都会降低 PAR 水平。 H460 细胞中的 DNA 修复和克隆形成受到 PARP-1 抑制的抑制。当 veliparib 与放射治疗联合使用时,H460 细胞表现出增加的隔离和自噬 [3]。在 H1299、DU145 和 22RV1 细胞中,veliparib 降低 PARP 活性;这种抑制与 p53 功能相关。在克隆 H1299 细胞中,veliparib (10 μM) 将四分之一分数 (SF) 减少 43%。在含氧的 H1299 细胞中,velibib 有效地分布辐射。在缺氧照射的细胞中,如 H1299、DU145 和 22RV1,veliparib 可以断开 SF[4]。
|
| 体内研究 (In Vivo) |
Veliparib 在小鼠、SD 喷雾剂、比格犬和食蟹猴中的颅后生物利用度为 56%–92%[1]。 veliparib(25 mg/kg,腹腔注射)可改善 NCI-H460 异种移植模型中的肿瘤生长延迟。与放射治疗联合使用时,Veliparib 可以减少肿瘤血管化[3]。 Veliparib,剂量为 3 和 12.5 mg/kg 时,使 A375 和 Colo829 异种移植模型的瘤内 PAR 水平降低了 95% 以上;这种抑制随着时间的推移是可逆的[4]。
Veliparib(ABT-888)在同基因黑色素瘤模型中增强替莫唑胺。[1] 替莫唑胺是新一代细胞毒性烷化剂,目前用于治疗中枢神经系统恶性肿瘤和黑色素瘤。替莫唑胺在小鼠和人类之间的药代动力学特征相似,这使得在与人类相似的暴露条件下对小鼠进行研究成为可能。这很重要,因为当细胞毒性药物的血浆药物浓度与人类相似时,临床前模型最能预测临床结果。因此,使用了50至62.5 mg/kg/d的替莫唑胺剂量,当通过浓度曲线下面积(AUC)或Cmax测量时,该剂量与临床相关剂量200 mg/m2(口服,q.d×5)的人体暴露量非常接近。在该剂量范围内,小鼠未观察到明显的毒性(如体重过度减轻、皮毛起皱、脱水等)。[1] B16模型对大多数化疗药物相对耐药,对替莫唑胺中度敏感,PARP抑制剂可以增强其敏感性Veliparib(ABT-888)口服后,以剂量依赖的方式显著增强替莫唑胺的疗效(图2A)。在第19天观察到最大增强,25、12.5和3.1 mg/kg/dVeliparib(ABT-888)组合组的T/C值(与替莫唑胺相比)分别为10(P=0.0003)、16(P<0.0001)和23(P<0.00001)。这些组合具有良好的耐受性,25mg/kg/d ABT-888和替莫唑胺组合的最大体重减轻11%,而替莫唑酰胺和ABT-888的最大体重减少7%和2%。给药期结束后,小鼠体重迅速回升。[1] 为了建立体内活动所需的稳态浓度,将Veliparib (ABT-888)与50mg/kg/d替莫唑胺联合连续输注。ABT-888在25至1mg/kg/d的剂量下均显著增强了替莫唑胺单一疗法(图2B)。25、12.5、5、1和0.3mg/kg/d ABT-888组合组在第17天出现最大增强,T/C值(与替莫唑胺相比)分别为13(P<0.0001)、12(P<0.0011)、16(P<0.00001)、39(P=0.0033)和63(不显著)。不能用替莫唑胺评估更高剂量的50 mg/kg/d ABT-888,因为这种组合会导致OMP植入部位的皮肤毒性。25和12.5mg/kg/d ABT-888联合治疗的活性是相等的,因此在该模型中,最大有效剂量定义为12.5mg/kg/d。与替莫唑胺增加5%相比,联合用药组的最大体重减轻为1%。[1] 体内抑制PAR。[1] PARP对DNA损伤的激活导致各种底物蛋白的核糖基化。为了显示体内PARP活性的抑制作用,通过蛋白质印迹分析用Veliparib(ABT-888)治疗的小鼠肿瘤的标准杆数水平。B16F10荷瘤小鼠分别用替莫唑胺单独或与ABT-888联合治疗(图2C),与疗效研究中使用的剂量相似(图2A)。观察到单独使用ABT-888或与替莫唑胺联合治疗的动物肿瘤中标准杆数蛋白的降低,表明ABT-888在体内抑制PARP活性的能力。 [1] Veliparib(ABT-888)在同基因胶质瘤模型中增强替莫唑胺。ABT-888还在9L原位大鼠胶质瘤模型中增强替莫唑胺。使用磁共振成像在第14天观察到最大疗效(图3A和C)。ABT-888 50 mg/kg/d联合替莫唑胺可将肿瘤体积减少63%,比单独使用替莫唑酰胺好44%(P<0.005)。ABT-888的肿瘤生长抑制具有剂量依赖性(图3A和B)。与替莫唑胺相比,50 mg/kg/d剂量的ABT-888与替莫唑胺的组合显著延长了动物的生存期,中位生存期分别为19天和22天(P<0.0132,时序检验;图3B)。ABT-888作为50mg/kg/d的单一药物在该模型中无效(数据未显示)。这些组合具有良好的耐受性,与替莫唑胺的8%相比,组合的最大体重减轻仅为9%。 Veliparib(ABT-888)增强铂剂。[1] 在MX-1乳腺癌异种移植物模型中研究了ABT-888增强铂类药物疗效的能力。这条线来自一名29岁的女性,她患有分化不良的乳腺癌。Abbott的内部测序工作已经确定MX-1具有BRCA1缺失。检测到一种新的BRCA1变体(BRCA1 33636delGAAA),该变体会导致移码突变,预计会引入链终止子并截断残基999处的蛋白质。在BRCA2中也检测到两种先前描述的非同义单核苷酸多态性(BRCA2 16864A>C,Asn289His和BRCA2 221847A>G,Asn991Asp),这两种多态性都已在中国癌症家族中描述。 Veliparib(ABT-888)诱导顺铂活性的显著增强(图4A)。在第68天,当所有小鼠仍存在于每个组合组中时,5、25和50mg/kg/d与顺铂的组合之间没有显著差异。然而,在试验结束时,ABT-888在5、25和50mg/kg/d的剂量下与顺铂联合使用,治愈率有所提高(分别为8/9、8/9和6/9只动物),而顺铂单药治疗仅能治愈3/9例(试验结束时没有可测量的肿瘤)。5和25mg/kg/d ABT-888联合顺铂与单独顺铂相比有显著差异(P=0.049,Fisher精确检验),而50mg/kg/d ABT-888与5和25mg/kg治疗组合没有显著差异。载体组和ABT-888单药治疗组没有治愈。这项剂量反应研究表明,ABT-888在5mg/kg/d时达到最大增强。在第二项使用卡铂的MX-1研究中证实了铂剂的增强作用。卡铂是第二代铂类,毒性较小的抗癌药物,目前是治疗肺癌、卵巢癌和头颈癌的标准治疗方法。通过OMPs以25mg/kg/d的剂量给药ABT-888,在10和15mg/kg/d时引起卡铂的明显增强,如肿瘤体积所示(图4B)。与第38天的卡铂治疗组相比,卡铂15和10mg/kg/d与ABT-888的组合的T/C值分别为34(P=0.011)和18(P<0.0001)。10mg/kg/d的卡铂/ABT-888组合从第26天开始使肿瘤体积缩小,而卡铂单一疗法仅具有适度的肿瘤抑制作用。 由于Veliparib (ABT-888)在10mg/kg/d时显示出明显的卡铂增强作用,因此进行了一项单独的研究,以确定固定卡铂剂量下ABT-888的剂量反应关系。对于ABT-888和卡铂的50、25和12.5 mg/kg/d组合,ABT-888在9天的第42天(P<0.0005)、22天(P=0.0014)和42天(P=0.012)增强了10 mg/kg/d卡铂的活性(q4d×3),T/C值百分比(与卡铂相比)(数据未显示)。5和1mg/kg/d剂量的ABT-888不会增强卡铂。 Veliparib (ABT-888)增强环磷酰胺的疗效。[1] 在MX-1模型中,通过OMPs以25mg/kg/d的剂量给药ABT-888,不仅在第20、24和27天以12.5mg/kg/d的浓度强化了环磷酰胺(图4C),还导致了肿瘤消退,而环磷酰胺单一疗法仅略微延迟了肿瘤生长。在第38天,这种组合(与环磷酰胺相比)的T/C值为35(P=0.0011)。环磷酰胺在5mg/kg/d时无效,ABT-888在该剂量下不能增强细胞毒性剂。在另一项验证性研究中,ABT-888以25mg/kg/d的剂量增强了环磷酰胺(12.5mg/kg/d,q4d×3)的疗效,但ABT-888在12.5mg/kg/d的剂量下没有显示出增强作用。25、12.5和5mg/kg/d剂量的ABT-888组合在第42天的T/C值(与卡铂相比)分别为53(P=0.018)、91(不显著)和100(不明显)(数据未显示) 在DOHH-2 B细胞淋巴瘤侧翼异种移植物模型中,还评估了Veliparib(ABT-888)增强环磷酰胺疗效的能力。DOHH-2是一种具有t(14;18)易位的淋巴瘤细胞系,导致Bcl-2的高水平表达,对环磷酰胺敏感。然而,尽管环磷酰胺显示出单药活性,ABT-888并没有使用一系列细胞毒性方案(q.d.1、q.d.4、q4d×2和q4d×3)和给药方案(数据未显示)来增强环磷酰胺。这些数据表明,ABT-888在DOHH-2模型中不是环磷酰胺的增效剂。 Veliparib (ABT-888)增强辐射。[1] HCT-116是一种人类结肠癌癌症系,其辐射敏感性已得到很好的表征,包括生长延迟、细胞周期阻滞和细胞凋亡。ABT-888通过OMPs以25mg/kg/d的剂量强化分级辐射(2 Gy/d×10天),中位生存期为36天,而单独辐射的中位生存时间为23天(P<0.036,时序检验)(图5)。尽管ABT-888在12.5mg/kg/d时没有提高中位生存期(34天)(P=0.06),但当研究在第65天终止时,该治疗组确实有一次治愈(没有可触及的肿瘤)。ABT-888也在5和1mg/kg/d的剂量下与辐射联合进行了测试,但这些组与单独辐射没有显著差异。总体而言,ABT-888与辐射联合显示出剂量反应(P=0.0165,对数秩趋势)。 |
| 酶活实验 |
在 PARP 检测中,50 mM Tris (pH 8.0)、1 mM DTT、1.5 μM [3H]NAD+ (1.6 μCi/mmol)、200 nM 生物素化组蛋白 H1将 200 nM slDNA 和 1 nM PARP-1 或 4 nM PARP-2 酶添加到缓冲液中。添加 1.5 mM 苯甲酰胺以终止反应后,将反应转移至链霉亲和素 Flash 板并使用 TopCount 微孔板闪烁计数器进行计数。
使用商业检测试剂盒测量 PARP1 酶活性;然而,我们没有使用试剂盒附带的 PARP1 蛋白,而是使用含有野生型 PARP1 或 PARP Y907 突变体的细胞裂解物。每个反应接收 500 ng 的全部裂解物。 Veliparib (ABT-888) 是一种 PARP 抑制剂,剂量范围为 0.01 至 1,000 μM。酶标仪用于测量野生型和突变型样品与底物孵育后的 PARP 酶活性[2]。 体外PARP和SIRT检测。PARP测定在含有50 mmol/L Tris(pH 8.0)、1 mmol/L DTT、1.5μmol/L[3H]NAD+(1.6μCi/mmol)、200 nmol/L生物素化组蛋白H1、200 nmol/L slDNA和1 nmol/L PARP-1或4 nmol/L PARP-2酶的缓冲液中进行。用1.5 mmol/L苯甲酰胺终止反应,转移到链霉抗生物素蛋白闪光板上,并使用TopCount微孔板闪烁计数器计数。[1] 烟酰胺[2,5′,8-3H]腺嘌呤二核苷酸和链霉抗生物素SPA珠购自xxx Biosciences。从大肠杆菌纯化的重组人PARP和6-生物素-17-NAD+购自xxx。NAD+、组蛋白、氨基苯甲酰胺、3-氨基苯甲胺和小牛胸腺DNA(dcDNA)来自xxx。茎环寡核苷酸(slDNA)CACAAGTTGCATTCCTC-TCTGAAGTTAAGACCTATGCAGAGAGAGAATGCAACACTTGTG是从Qiagen获得的,含有MCAT序列(斜体)。将寡核苷酸溶解在含有10 mmol/L Tris-HCl(pH 7.5)、1 mmol/L EDTA和50 mmol/L NaCl的退火缓冲液中,在95°C下孵育5分钟,然后在45°C下退火45分钟。组蛋白H1(95%电泳纯)购自yyy。通过用磺基-NHS LC生物素处理蛋白质制备生物素化组蛋白H1。SIRT2测定如前所述进行。 |
| 细胞实验 |
细胞PARP检测[2]
C41细胞在96孔板中用试验化合物处理30分钟。通过用1 mM H2O2损伤DNA 10分钟来激活PARP。细胞用冰冷的PBS洗涤一次,并在-20°C下用预冷的甲醇/丙酮(7:3)固定10分钟。空气干燥后,用PBS对平板进行再水化,并在室温下用PBS中的5%脱脂奶粉(0.05%)(封闭溶液)封闭30分钟。将细胞与抗PAR抗体10H(1:50)在封闭溶液中在室温下孵育60分钟,然后用PBS-Tween20洗涤5次,并与山羊抗小鼠荧光素5(6)-异硫氰酸酯(FITC)-偶联抗体(1:100)和1μg/mL 4′,6-二脒基-2-苯基吲哚(DAPI)在封闭液中在室温孵育60 min。用PBS-Teen20洗涤五次后,使用设置为FITC的激发和发射波长或DAPI的激发和辐射波长的fmax荧光微孔板读取器进行分析。PARP活性(FITC信号)用细胞数(DAPI)归一化。 |
| 动物实验 |
In order to conduct syngeneic studies on B16F10, a mixture of 6×104 cells and 50% Matrigel is injected subcutaneously (20 g) into the flank of 6- to 8-week-old female C57BL/6 mice. In order to conduct cisplatin efficacy studies, fragments (20–30 mm3) of human tumors taken from spontaneously growing tumors in nude mouse hosts are trocar-implanted in female nude mice. In the studies involving carboplatin and MX-1 cyclophosphamide, 200 μL of a 1:10 dilution of tumor brei in 45% Matrigel and 45% Spinner MEM is administered to female scid mice as an injection. Tumors are allowed to grow to the specified size in these well-established tumor studies, after which they are randomized to therapy groups. Male scid mice are given a s.c. injection of 1×106 cells mixed with 50% Matrigel to be used in DOHH-2 xenograft studies. Veliparib is administered orally or continuously via s.c. insertion of a 14-day Alzet OMP model 2002 in a pH 4.0-adjusted 0.9% NaCl solution. Doses of Veliparib are determined based on the OMP's daily delivery rate of 12 μL. The formulation of temozolomide, cisplatin, carboplatin, and cyclophosphamide is done in accordance with the advice of the manufacturers.
|
| 药代性质 (ADME/PK) |
Pharmacokinetics Results [5]
PK parameters in the group with dosages greater than or equal to 200 mg BID (high veliparib, n=18) and those less than 200 mg BID (low veliparib, n=7) were compared, utilizing an unpaired, 2 sided t-test. PK studies were performed on 25 of 31 patients with complete samples. We noted a positive correlation between the AUC/mg of PLD (area under the curve on a plot of drug concentration in blood plasma vs time) and veliparib dose, p=0.001, and a negative correlation between PLD clearance (CL), p=0.001 and veliparib dose. When analyzed as low and high veliparib groups, the mean (SD) half-life (hrs) was significantly lower in the low veliparib when compared to the high veliparib group, 83.2 (33.2) vs 108.6 (24.88) (p =0.042), and the mean PLD clearance (mL/h) was greater in the low veliparib versus the high veliparib group 35.9 (15.9) vs 14.2 (4.3), P<.0001. Similarly, the PLD AUC/mg dose (mg x h/L) was significantly lower in the low vs high veliparib groups, 35.0 (14.7)vs 74.9 (18.3), P<0.0001. Thirteen patients had evaluable veliparib PK from the expanded cohort (see supplement). The half-life was slightly shorter at 4.1 h on day 1 compared to 5.3 h on day 8. The day 8 apparent clearance was 15.4 L/h and apparent volume of distribution was 121 L with both parameters calculated using day 8 AUC0–12. Dose-normalizing exposure to accommodate dose reductions between days 1 and 8 resulted in statistical significance of day 1 Cmax vs predicted day 8 Cmax (Fig. 3A, p-value=0.0022) and day 1 AUC0−∞ vs day 8 AUC0–12 (Fig 3B, p-value 0.0151). Observed accumulation ratios were lower than the expected 1.2 accumulation ratio that was calculated with the observed half-life and 12 h dosing interval. The observed accumulation ratio for Cmax was 0.865, AUC0–12 was 0.816, and AUC0–12/ AUC0−∞ was 0.666. |
| 毒性/毒理 (Toxicokinetics/TK) |
The majority of patients (39%) were treated at 200 mg of veliparib BID (final dose reached after de-escalation) and 70% were treated with 40 mg/m2 PLD. Dose de-escalation of veliparib or PLD occurred due to adverse events in 20 (49%) patients in stratum B and 2 (67%) in stratum A, including patients with a DLT. Table 3 shows adverse events. Although grade 1 and 2 toxicities were common, only 10% patients experienced a grade 3 or 4 toxicity from this therapy regimen. The most common grade 3 and 4 adverse events included: anemia, 4 (10%); neutropenia, 4 (10%); hand-foot syndrome (HFS), 4 (10%); nausea, 2 (5%); vomiting, 2 (5%); neuropathy, 2 (5%); and transaminitis, 2 (5%). Therapy was discontinued for 5 (11%) patients due to toxicity. In stratum A, a patient developed grade 4 pericardial tamponade at 200 mg veliparib BID and 40 mg/m2 PLD (after 1 cycle). Cytology from the pericardial fluid was positive for malignant cells. In stratum B therapy was discontinued due unresolved thrombocytopenia at 50 mg veliparib BID and 22.5 mg/m2 PLD (after 24 cycles), grade 3 HFS at 50 mg veliparib BID and 22.5 mg/m2 PLD (after 32 cycles), a decreased left ventricular ejection fraction (52%, vs 72% LVEF prior to treatment) at 200 mg veliparib BID and 40 mg/m2 PLD (after 5 cycles), and unresolved leukopenia at 300 mg veliparib BID and 40 mg/m2 PLD (after 2 cycles).[5]
|
| 参考文献 |
|
| 其他信息 |
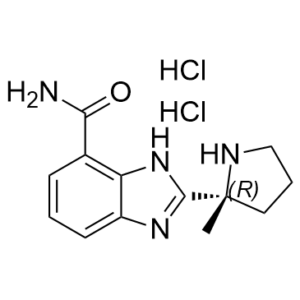
Veliparib is a benzimidazole substituted with a carbamoyl group at C-4 and a (2R)-2-methylpyrrolidin-2-yl moiety at C-2. It is a potent, orally bioavailable PARP inhibitor. It has a role as an EC 2.4.2.30 (NAD(+) ADP-ribosyltransferase) inhibitor.
<
Veliparib is a poly(ADP-ribose) polymerase (PARP) -1 and -2 inhibitor with chemosensitizing and antitumor activities. With no antiproliferative effects as a single agent at therapeutic concentrations, ABT-888 inhibits PARPs, thereby inhibiting DNA repair and potentiating the cytotoxicity of DNA-damaging agents. PARP nuclear enzymes are activated by DNA single or double strand breaks, resulting in the poly(ADP-ribosyl)ation of other nuclear DNA binding proteins involved in DNA repair; poly(ADP-ribosyl)ation contributes to efficient DNA repair and to survival of proliferating cells exposed to mild genotoxic stresses as induced by as oxidants, alkylating agents or ionizing radiation. Drug Indication Treatment of high-grade glioma; Treatment of fallopian tube cancer , Treatment of ovarian cancer , Treatment of peritoneal carcinoma;Treatment of lung carcinoma (small cell and non-small cell carcinoma) Veliparib is a benzimidazole substituted with a carbamoyl group at C-4 and a (2R)-2-methylpyrrolidin-2-yl moiety at C-2. It is a potent, orally bioavailable PARP inhibitor. It has a role as an EC 2.4.2.30 (NAD(+) ADP-ribosyltransferase) inhibitor. Veliparib is a poly(ADP-ribose) polymerase (PARP) -1 and -2 inhibitor with chemosensitizing and antitumor activities. With no antiproliferative effects as a single agent at therapeutic concentrations, ABT-888 inhibits PARPs, thereby inhibiting DNA repair and potentiating the cytotoxicity of DNA-damaging agents. PARP nuclear enzymes are activated by DNA single or double strand breaks, resulting in the poly(ADP-ribosyl)ation of other nuclear DNA binding proteins involved in DNA repair; poly(ADP-ribosyl)ation contributes to efficient DNA repair and to survival of proliferating cells exposed to mild genotoxic stresses as induced by as oxidants, alkylating agents or ionizing radiation. Drug Indication Treatment of high-grade glioma Treatment of fallopian tube cancer , Treatment of ovarian cancer , Treatment of peritoneal carcinoma Treatment of lung carcinoma (small cell and non-small cell carcinoma) Treatment of breast cancer. Purpose: To evaluate the preclinical pharmacokinetics and antitumor efficacy of a novel orally bioavailable poly(ADP-ribose) polymerase (PARP) inhibitor, ABT-888. Experimental design: In vitro potency was determined in a PARP-1 and PARP-2 enzyme assay. In vivo efficacy was evaluated in syngeneic and xenograft models in combination with temozolomide, platinums, cyclophosphamide, and ionizing radiation. Results: ABT-888 is a potent inhibitor of both PARP-1 and PARP-2 with K(i)s of 5.2 and 2.9 nmol/L, respectively. The compound has good oral bioavailability and crosses the blood-brain barrier. ABT-888 strongly potentiated temozolomide in the B16F10 s.c. murine melanoma model. PARP inhibition dramatically increased the efficacy of temozolomide at ABT-888 doses as low as 3.1 mg/kg/d and a maximal efficacy achieved at 25 mg/kg/d. In the 9L orthotopic rat glioma model, temozolomide alone exhibited minimal efficacy, whereas ABT-888, when combined with temozolomide, significantly slowed tumor progression. In the MX-1 breast xenograft model (BRCA1 deletion and BRCA2 mutation), ABT-888 potentiated cisplatin, carboplatin, and cyclophosphamide, causing regression of established tumors, whereas with comparable doses of cytotoxic agents alone, only modest tumor inhibition was exhibited. Finally, ABT-888 potentiated radiation (2 Gy/d x 10) in an HCT-116 colon carcinoma model. In each model, ABT-888 did not display single-agent activity. Conclusions: ABT-888 is a potent inhibitor of PARP, has good oral bioavailability, can cross the blood-brain barrier, and potentiates temozolomide, platinums, cyclophosphamide, and radiation in syngeneic and xenograft tumor models. This broad spectrum of chemopotentiation and radiopotentiation makes this compound an attractive candidate for clinical evaluation. [1] View MoreTo evaluate the preclinical pharmacokinetics and antitumor efficacy of a novel orally bioavailable poly(ADP-ribose) polymerase (PARP) inhibitor, ABT-888. Experimental design: In vitro potency was determined in a PARP-1 and PARP-2 enzyme assay. In vivo efficacy was evaluated in syngeneic and xenograft models in combination with temozolomide, platinums, cyclophosphamide, and ionizing radiation. Results: ABT-888 is a potent inhibitor of both PARP-1 and PARP-2 with K(i)s of 5.2 and 2.9 nmol/L, respectively. The compound has good oral bioavailability and crosses the blood-brain barrier. ABT-888 strongly potentiated temozolomide in the B16F10 s.c. murine melanoma model. PARP inhibition dramatically increased the efficacy of temozolomide at ABT-888 doses as low as 3.1 mg/kg/d and a maximal efficacy achieved at 25 mg/kg/d. In the 9L orthotopic rat glioma model, temozolomide alone exhibited minimal efficacy, whereas ABT-888, when combined with temozolomide, significantly slowed tumor progression. In the MX-1 breast xenograft model (BRCA1 deletion and BRCA2 mutation), ABT-888 potentiated cisplatin, carboplatin, and cyclophosphamide, causing regression of established tumors, whereas with comparable doses of cytotoxic agents alone, only modest tumor inhibition was exhibited. Finally, ABT-888 potentiated radiation (2 Gy/d x 10) in an HCT-116 colon carcinoma model. In each model, ABT-888 did not display single-agent activity. Conclusions: ABT-888 is a potent inhibitor of PARP, has good oral bioavailability, can cross the blood-brain barrier, and potentiates temozolomide, platinums, cyclophosphamide, and radiation in syngeneic and xenograft tumor models. This broad spectrum of chemopotentiation and radiopotentiation makes this compound an attractive candidate for clinical evaluation.[1] Poly (ADP-ribose) polymerase (PARP) inhibitors have emerged as promising therapeutics for many diseases, including cancer, in clinical trials. One PARP inhibitor, olaparib (Lynparza, AstraZeneca), was recently approved by the FDA to treat ovarian cancer with mutations in BRCA genes. BRCA1 and BRCA2 have essential roles in repairing DNA double-strand breaks, and a deficiency of BRCA proteins sensitizes cancer cells to PARP inhibition. Here we show that the receptor tyrosine kinase c-Met associates with and phosphorylates PARP1 at Tyr907 (PARP1 pTyr907 or pY907). PARP1 pY907 increases PARP1 enzymatic activity and reduces binding to a PARP inhibitor, thereby rendering cancer cells resistant to PARP inhibition. The combination of c-Met and PARP1 inhibitors synergized to suppress the growth of breast cancer cells in vitro and xenograft tumor models, and we observed similar synergistic effects in a lung cancer xenograft tumor model. These results suggest that the abundance of PARP1 pY907 may predict tumor resistance to PARP inhibitors, and that treatment with a combination of c-Met and PARP inhibitors may benefit patients whose tumors show high c-Met expression and who do not respond to PARP inhibition alone.[2] Early studies with first-generation poly (ADP-ribose) polymerase (PARP) inhibitors have already indicated some therapeutic potential for sulfur mustard (SM) injuries. The available novel and more potential PARP inhibitors, which are undergoing clinical trials as drugs for cancer treatment, bring it back to the centre of interest. However, the role of PARP-1 in SM-induced injury is not fully understood. In this study, we selected a high potent specific PARP inhibitor ABT-888 as an example to investigate the effect of PARP inhibitor in SM injury. The results showed that in both the mouse ear vesicant model (MEVM) and HaCaT cell model, PARP inhibitor ABT-888 can reduce cell damage induced by severe SM injury. ABT-888 significantly reduced SM induced edema and epidermal necrosis in MEVM. In the HaCaT cell model, ABT-888 can reduce SM-induced NAD(+)/ATP depletion and apoptosis/necrosis. Then, we studied the mechanism of PARP-1 in SM injury by knockdown of PARP-1 in HaCaT cells. Knockdown of PARP-1 protected cell viability and downregulated the apoptosis checkpoints, including p-JNK, p-p53, Caspase 9, Caspase 8, c-PARP and Caspase 3 following SM-induced injury. Furthermore, the activation of AKT can inhibit autophagy via the regulation of mTOR. Our results showed that SM exposure could significantly inhibit the activation of Akt/mTOR pathway. Knockdown of PARP-1 reversed the SM-induced suppression of the Akt/mTOR pathway. In summary, the results of our study indicated that the protective effects of downregulation of PARP-1 in SM injury may be due to the regulation of apoptosis, necrosis, energy crisis and autophagy. However, it should be noticed that PARP inhibitor ABT-888 further enhanced the phosphorylation of H2AX (S139) after SM exposure, which indicated that we should be very careful in the application of PARP inhibitors in SM injury treatment because of the enhancement of DNA damage.[3] |
| 分子式 |
C13H18CL2N4O
|
|
|---|---|---|
| 分子量 |
317.21
|
|
| 精确质量 |
316.085
|
|
| 元素分析 |
C, 49.22; H, 5.72; Cl, 22.35; N, 17.66; O, 5.04
|
|
| CAS号 |
912445-05-7
|
|
| 相关CAS号 |
912444-00-9; 912445-05-7 (HCl)
|
|
| PubChem CID |
45480520
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| LogP |
3
|
|
| tPSA |
4.077
|
|
| 氢键供体(HBD)数目 |
5
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
20
|
|
| 分子复杂度/Complexity |
348
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
C(C1C=CC=C2N=C([C@@]3(NCCC3)C)NC=12)(=O)N.Cl
|
|
| InChi Key |
DSBSVDCHFMEYBX-FFXKMJQXSA-N
|
|
| InChi Code |
InChI=1S/C13H16N4O.2ClH/c1-13(6-3-7-15-13)12-16-9-5-2-4-8(11(14)18)10(9)17-12;;/h2,4-5,15H,3,6-7H2,1H3,(H2,14,18)(H,16,17);2*1H/t13-;;/m1../s1
|
|
| 化学名 |
2-[(2R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide;dihydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 100 mg/mL (315.25 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1525 mL | 15.7624 mL | 31.5249 mL | |
| 5 mM | 0.6305 mL | 3.1525 mL | 6.3050 mL | |
| 10 mM | 0.3152 mL | 1.5762 mL | 3.1525 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03581292 | Active Recruiting |
Drug: Veliparib Drug: Temozolomide |
Glioblastoma (AML) Malignant Glioma |
National Cancer Institute (NCI) |
November 6, 2018 | Phase 2 |
| NCT02163694 | Active Recruiting |
Drug: Veliparib Drug: Veliparib Placebo |
Metastatic Breast Cancer | AbbVie | July 17, 2014 | Phase 3 |
| NCT02595905 | Active Recruiting |
Drug: Veliparib Drug: Cisplatin |
Metastatic Breast Carcinoma Recurrent Breast Carcinoma |
National Cancer Institute (NCI) |
September 15, 2016 | Phase 2 |
| NCT02631733 | Active Recruiting |
Drug: Ferumoxytol Drug: Veliparib |
Malignant Solid Neoplasm | National Cancer Institute (NCI) |
May 31, 2017 | Phase 1 |
| NCT01434316 | Active Recruiting |
Drug: Veliparib Drug: Dinaciclib |
Advanced Malignant Solid Neoplasm | National Cancer Institute (NCI) |
November 1, 2011 | Phase 1 |