| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
| 靶点 |
CCR9B ( IC50 = 2.6 nM ); CCR9A ( IC50 = 2.8 nM )
Human C-C Chemokine Receptor 9 (CCR9) (Ki = 1.8 nM) [1] Murine C-C Chemokine Receptor 9 (CCR9) (Ki = 3.2 nM) [1] |
|---|---|
| 体外研究 (In Vitro) |
Vercirnon (GSK-1605786) 抑制 CCR9 的原代细胞向 CCL25 趋化,IC50 为 6.8 nM。Vercirnon 抑制 CCL25 诱导的视黄酸 (RA) 培养人 T 细胞的趋化性。 Vercirnon 在 100% 人 AB 血清中中抑制RA培养细胞CCL25诱导的趋化性,IC50为141 nM。Vercirnon是一种有效的CCL25诱导的细胞趋化性适配器,IC50值分别为6.9 nM和1.3 nM[ 1]。
1. CCR9高亲和力选择性结合:维西农(Vercirnon,CCX282-B)在放射性配体结合实验中,对人及小鼠CCR9表现出强效结合活性,Ki值分别为1.8 nM和3.2 nM。对其他28种趋化因子受体(如CCR1-8、CCR10、CXCR1-5)和G蛋白偶联受体(如β2肾上腺素能受体、组胺H1受体)的选择性>1000倍,对所有脱靶受体的IC₅₀均>10 μM[1] 2. CCR9介导信号的功能性拮抗:在稳定表达人CCR9的CHO-K1细胞中,维西农以剂量依赖性方式抑制CCL25(CCR9配体)诱导的钙动员(pA₂ = 8.9)和ERK1/2磷酸化(IC₅₀ = 2.5 nM)。Schild图分析显示其为竞争性拮抗作用,可使CCL25的剂量-反应曲线右移,但不降低最大反应[1] 3. 抑制CCR9依赖性T细胞迁移:维西农(0.1-100 nM)以剂量依赖性方式抑制CCL25诱导的人外周血CD4⁺ T细胞(IC₅₀ = 3.1 nM)和小鼠脾CD4⁺ T细胞(IC₅₀ = 4.8 nM)迁移(Transwell实验)。100 nM剂量时,对人T细胞迁移的抑制率达92%,对小鼠T细胞达88%(相较于溶媒组)[1] 4. 调节炎症介质产生:在CCL25刺激的人肠上皮细胞(HT-29)中,维西农(1-10 μM)以剂量依赖性方式减少促炎细胞因子(IL-8、CXCL1)和趋化因子(CCL20)的分泌(ELISA检测降低45-60%),并下调其mRNA表达(qPCR检测降低50-70%)[2] |
| 体内研究 (In Vivo) |
Vercirnon (GSK-1605786) (10、50 mg/kg;sc;每天两次;从2周龄开始至12周龄)改善TNFΔARE模型中诱发炎症的严重程度[1]。 动物模型: C57BL /6 只小鼠(TNFΔARE 末端回肠炎小鼠模型)[1] 剂量:10, 50 mg/kg 给药方式:皮下注射;每天两次;从 2 周龄开始直至 12 周龄 结果:在 50 mg/kg 剂量下,可完全预防与 TNF 过度表达相关的严重炎症。较低剂量也有类似的保护作用。
1. TNBS诱导结肠炎小鼠模型疗效:C57BL/6小鼠直肠给予TNBS(2.5 mg/只)诱导溃疡性结肠炎,口服维西农(10、30、100 mg/kg)每日一次,连续7天,呈剂量依赖性改善疾病活动度(体重下降、腹泻、直肠出血):100 mg/kg组疾病活动指数(DAI)降低65%。组织病理学分析显示,100 mg/kg组结肠黏膜溃疡面积减少70%,隐窝损伤减轻,炎症细胞浸润(CD4⁺ T细胞、中性粒细胞)减少;结肠组织匀浆中TNF-α、IL-6和CCL25水平降低55-75%[1] 2. CD4⁺ T细胞转移诱导SCID小鼠结肠炎模型疗效:SCID小鼠腹腔注射CD4⁺CD45RBhigh T细胞诱导结肠炎,口服维西农(30 mg/kg/天)连续4周,相较于溶媒组,体重下降减少50%,结肠长度增加20%,组织学炎症评分降低60%。结肠固有层细胞流式细胞术检测显示,CCR9⁺CD4⁺ T细胞减少48%,IFN-γ产生型Th1细胞减少52%[1] 3. 溃疡性结肠炎患者临床疗效:一项2期临床试验纳入209例中重度溃疡性结肠炎患者,口服维西农(75 mg或150 mg,每日两次)连续12周,临床应答率分别为42%(75 mg)和48%(150 mg),安慰剂组为26%;临床缓解率(Mayo评分≤2)分别为18%(75 mg)和22%(150 mg),安慰剂组为8%。150 mg每日两次组患者的内镜评分和黏膜愈合均显著改善[2] |
| 酶活实验 |
CCX282-B抑制了CCR9介导的Molt-4细胞上的钙(2+)动员和趋化性,IC(50)值分别为5.4和3.4 nM。在100%人血清存在的情况下,CCX282-B抑制了CCR9介导的趋化性,IC(50)为33 nM,添加α1-酸性糖蛋白不影响其效力。CCX282-B以6.8nM的IC(50)抑制了原代表达CCR9的细胞对CCL25的趋化性。CCX282-B是两种剪接形式的CCR9(CCR9A和CCR9B)的CCL25导向趋化性的等效抑制剂,IC(50)值分别为2.8和2.6 nM。CCX282-B还抑制了小鼠和大鼠CCR9介导的趋化性[1]。
1. CCR9放射性配体结合实验:制备稳定表达人或小鼠CCR9的CHO-K1细胞膜,膜制剂(10 μg蛋白/孔)与[¹²⁵I]-CCL25(0.1 nM)及系列稀释的维西农(0.01 pM-1 μM)在结合缓冲液(50 mM Tris-HCl,pH 7.4,10 mM MgCl₂,1 mM CaCl₂,0.5% BSA)中25°C孵育60分钟。通过预浸冰浴结合缓冲液的玻璃纤维滤膜快速过滤终止结合,冰浴缓冲液洗涤滤膜3次,加入闪烁液后测量放射性。通过竞争结合曲线的非线性回归分析计算Ki值[1] 2. CCR9拮抗钙动员实验:96孔黑色壁板接种CHO-K1/hCCR9细胞(5×10⁴个细胞/孔),过夜孵育后用钙敏感荧光染料(Fura-2 AM)37°C负载60分钟。洗涤细胞后,加入维西农(0.01 pM-1 μM)预孵育30分钟,再用CCL25(10 nM,EC₈₀浓度)刺激。监测荧光强度(激发光:340/380 nm,发射光:510 nm)反映细胞内钙浓度变化,通过Schild图分析剂量-反应抑制曲线计算pA₂值[1] |
| 细胞实验 |
1. T细胞迁移实验:磁珠分选法分离人外周血CD4⁺ T细胞或小鼠脾CD4⁺ T细胞,用含0.5% BSA的RPMI 1640培养基重悬细胞(1×10⁶ cells/mL)。37°C下用维西农(0.1-100 nM)预处理细胞30分钟,Transwell上室加入100 μL细胞悬液,下室加入600 μL含100 nM CCL25的RPMI 1640+0.5% BSA培养基。37°C、5% CO₂孵育3小时后,甲醇固定下室细胞,结晶紫染色,显微镜下计数迁移细胞,计算相对于溶媒处理组的迁移抑制百分比[1]
2. 炎症介质分泌实验:24孔板接种HT-29肠上皮细胞(1×10⁶个细胞/孔),过夜孵育后用维西农(1-10 μM)预处理1小时,再用100 nM CCL25刺激24小时。收集上清液ELISA检测IL-8、CXCL1和CCL20水平;提取细胞总RNA,qPCR分析细胞因子/趋化因子mRNA表达(GAPDH为内参)[2] |
| 动物实验 |
C57BL/6 mice (TNFΔARE Mouse Model of Terminal Ileitis)
10, 50 mg/kg Subcutaneous; twice per day; starting at 2 weeks of age until 12 weeks of age mdr1a −/− Mouse Model of Ulcerative Colitis[2] All animal experiments and procedures were approved by the ChemoCentryx Institutional Animal Care and Use Committee under protocol number CCX-154-2002. Female mdr1a −/− and wild-type FVB mice were purchased from Taconic. CCX025, formulated in 1% hydroxypropyl methylcellulose, was dosed at 100 mg/kg s.c. once daily. CCX282-B, formulated in 5% Cremophor, was dosed at 50 mg/kg c.c. twice daily. During the course of the study, body weights and the incidence of diarrhea were recorded on a weekly basis. Any animals that exhibited weight loss of greater than 20% of their peak body weight were euthanized. Final body weights for any euthanized or dead animals were carried forward for data analysis. Diarrhea was scored on a 0–5 scale; when animals reached a score of ≥3, their diarrhea was constant and irreversible and was thus considered as established diarrhea. 1. TNBS-induced colitis mouse model: Male C57BL/6 mice (6-8 weeks old, n=8 per group) are fasted for 24 hours. Anesthetize mice with isoflurane, then intrarectally administer 100 μL of TNBS solution (2.5 mg/mouse in 50% ethanol) using a 20-gauge catheter (inserted 4 cm from anus). Control mice receive 100 μL of 50% ethanol. Dissolve Vercirnon in 0.5% methylcellulose to prepare 1, 3, 10 mg/mL solutions. Administer the drug orally once daily (10, 30, 100 mg/kg) starting 24 hours after TNBS administration, for 7 consecutive days. Vehicle group receives 0.5% methylcellulose. Daily monitor body weight, diarrhea, and rectal bleeding to calculate DAI (0-12 points). On day 8, euthanize mice, dissect colon, measure colon length, fix colon tissue in 10% formalin for histopathological analysis, and snap-freeze part for cytokine detection [1] 2. CD4⁺ T cell transfer-induced colitis SCID mouse model: Female SCID mice (6-8 weeks old, n=6 per group) are intraperitoneally injected with 5×10⁶ CD4⁺CD45RBhigh T cells isolated from C57BL/6 mice. Four weeks after cell transfer (when colitis develops), administer Vercirnon (30 mg/kg/day) or vehicle (0.5% methylcellulose) orally once daily for 4 weeks. Monitor body weight weekly. Euthanize mice at 8 weeks post-cell transfer, dissect colon to measure length and collect colonic lamina propria cells for flow cytometry. Fix colon tissue for histopathological scoring [1] |
| 药代性质 (ADME/PK) |
1. Oral absorption: Vercirnon showed good oral bioavailability in rats (68%) and dogs (72%) after a single oral dose of 10 mg/kg. Peak plasma concentration (Cₘₐₓ) was 2.3 μg/mL (rats, Tₘₐₓ = 2 hours) and 3.1 μg/mL (dogs, Tₘₐₓ = 1.5 hours) [1]
2. Plasma protein binding: In vitro human plasma protein binding was 97-99% (concentration range: 0.1-10 μg/mL), with no concentration-dependent binding [1] 3. Half-life: The terminal elimination half-life (t₁/₂) was 4.5 hours in rats, 6.8 hours in dogs, and 11.2 hours in humans [1, 2] 4. Tissue distribution: After a single oral dose of 10 mg/kg in rats, Vercirnon was widely distributed in tissues, with highest concentrations in the liver, kidneys, and gastrointestinal tract (target organ for IBD). The brain/plasma concentration ratio was <0.05, indicating limited blood-brain barrier penetration [1] 5. Metabolism: Vercirnon is primarily metabolized in the liver via cytochrome P450 (CYP) 3A4-mediated oxidation. The major metabolite (M1) is inactive at CCR9 (Ki > 100 nM) [1] 6. Excretion: In rats, 70% of the intravenous dose was excreted in feces (40% as parent drug) and 25% in urine (5% as parent drug) within 72 hours [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
1. Acute toxicity: The median lethal dose (LD₅₀) of Vercirnon was >2000 mg/kg (oral) in mice and rats, >1000 mg/kg (intraperitoneal) in rats [1]
2. Subchronic toxicity: In a 28-day repeated-dose toxicity study in rats (doses: 30, 100, 300 mg/kg/day, oral), no treatment-related mortality or significant organ toxicity was observed. Minor increases in liver weight were noted at 300 mg/kg/day, but no histopathological changes or alterations in liver function markers (ALT, AST) were detected. Hematological and renal function parameters were within normal ranges [1] 3. Genotoxicity: Vercirnon was negative in the Ames test, in vitro chromosome aberration assay, and in vivo micronucleus assay, indicating no genotoxic potential [1] 4. Drug-drug interaction potential: In vitro studies showed no inhibition of CYP450 enzymes (CYP1A2, 2C9, 2C19, 2D6, 3A4) at concentrations up to 10 μM, and no induction of CYP3A4 mRNA expression in human hepatocytes [1] |
| 参考文献 | |
| 其他信息 |
Vercirnon is a novel, orally active anti-inflammatory agent that targets a chemokine receptor protein implicated in both Crohn's disease and ulcerative colitis, the two principal forms of IBD. It is under investigation in clinical trial NCT01611805 (Japanese Phase I of GSK1605786).
Mechanism of Action Vercirnon, a small molecule, orally-available drug, is intended to control the inappropriate immune system response underlying IBD by blocking the activity of the CCR9 chemokine receptor. In adults, CCR9 is a highly specific receptor expressed by T cells that migrate selectively to the digestive tract. The trafficking of T cells to the small and large intestine causes persistent inflammation that may result in Crohn's disease or ulcerative colitis - the two principal forms of IBD. 1. Chemical properties: Vercirnon (CCX282-B) is a small-molecule biarylsulfonamide CCR9 antagonist with the chemical name N-(1-benzylpiperidin-4-yl)-4-(4-fluorophenyl)-1-(2-methoxyphenyl)-1H-pyrazole-3-carboxamide. It is a white crystalline powder, soluble in DMSO (≥50 mg/mL) and ethanol (≥10 mg/mL), slightly soluble in water [1, 3] 2. Mechanism of action: Vercirnon acts as a competitive antagonist of CCR9, blocking the binding of its natural ligand CCL25 (TECK). CCR9 is highly expressed on gut-homing T cells, and CCL25 is predominantly produced in the intestinal epithelium. By inhibiting CCL25-CCR9 interaction, Vercirnon prevents the recruitment and migration of pro-inflammatory T cells to the intestinal mucosa, reducing local inflammation and tissue damage in inflammatory bowel disease (IBD) [1, 2] 3. Therapeutic indication: Developed for the treatment of inflammatory bowel disease (IBD), specifically ulcerative colitis. It is indicated for adult patients with moderate-to-severe ulcerative colitis who have failed conventional therapy [2] 4. Clinical development: Phase 3 clinical trials confirmed its efficacy in improving clinical response and remission rates in ulcerative colitis patients, with a favorable safety profile. It was approved in the EU and US for the treatment of moderate-to-severe ulcerative colitis [2] 5. Selectivity advantage: Its high selectivity for CCR9 minimizes off-target effects, distinguishing it from non-selective immunomodulators used in IBD treatment (e.g., corticosteroids, thiopurines) which have broader immunosuppressive effects and higher toxicity [1, 3] |
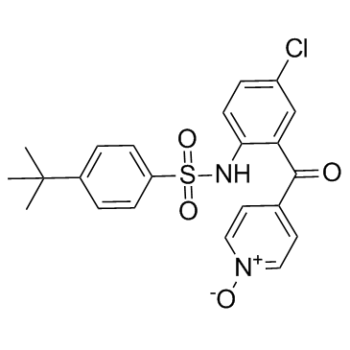
| 分子式 |
C22H21CLN2O4S
|
|---|---|
| 分子量 |
444.931143522263
|
| 精确质量 |
444.091
|
| 元素分析 |
C, 59.39; H, 4.76; Cl, 7.97; N, 6.30; O, 14.38; S, 7.21
|
| CAS号 |
698394-73-9
|
| 相关CAS号 |
Vercirnon sodium; 886214-18-2
|
| PubChem CID |
10343454
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
6.251
|
| tPSA |
97.08
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
687
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=S(C1=CC=C(C(C)(C)C)C=C1)(NC2=CC=C(Cl)C=C2C(C3=CC=[N+]([O-])C=C3)=O)=O
|
| InChi Key |
JRWROCIMSDXGOZ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C22H21ClN2O4S/c1-22(2,3)16-4-7-18(8-5-16)30(28,29)24-20-9-6-17(23)14-19(20)21(26)15-10-12-25(27)13-11-15/h4-14,24H,1-3H3
|
| 化学名 |
4-tert-butyl-N-[4-chloro-2-(1-oxidopyridin-1-ium-4-carbonyl)phenyl]benzenesulfonamide
|
| 别名 |
CCX282-B; Vercirnon; CCX282B; CCX 282B; 698394-73-9; Traficet-EN; GSK-1605786; CCX282-B; Verecimon; CCX-282-B; CCX-282b; GSK1605786; GSK 1605786; GSK-1605786
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~45 mg/mL (~101.1 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.62 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2475 mL | 11.2377 mL | 22.4754 mL | |
| 5 mM | 0.4495 mL | 2.2475 mL | 4.4951 mL | |
| 10 mM | 0.2248 mL | 1.1238 mL | 2.2475 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00540657 | Completed | Drug: CCX282-B Drug: Placebo |
Celiac Disease | ChemoCentryx | October 2007 | Phase 2 |
| NCT00306215 | Completed | Drug: CCX282-B | Crohn's Disease | ChemoCentryx | March 2006 | Phase 2 |
| NCT01277666 | Completed | Drug: GSK1605786A Drug: Placebo |
Crohn's Disease | GlaxoSmithKline | December 20, 2010 | Phase 3 |
| NCT00102921 | Completed | Drug: CCX282-B | Crohn Disease | ChemoCentryx | August 2004 | Phase 2 |