| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| Other Sizes |
|
| 靶点 |
Alpha-1A adrenergic receptor ( Ki = 0.05 μM ); Alpha-1B adrenergic receptor ( Ki = 0.30 μM ); Alpha-1D adrenergic receptor ( Ki = 0.15 μM ); Alpha-2A adrenergic receptor ( Ki = 0.88 μM ); Alpha-2B adrenergic receptor ( Ki = 1.7 μM ); Alpha-2C adrenergic receptor ( Ki = 0.19 μM nM )
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:Xylometazoline 是一种 α-肾上腺素受体激动剂,通常用作鼻减充血剂,对 α2B-肾上腺素受体亚型表现出最高效力,EC50 为 99 μM。 Xylometazoline 与肾上腺素受体亚型 α1A、α1B、α1D、α2A、α2B、α2C 结合,IC50 分别为 0.08、0.56、0.45、0.98、1.8、0.22μM。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
No information is available on xylometazoline pharmacokinetics. No information is available on xylometazoline pharmacokinetics. No information is available on xylometazoline pharmacokinetics. No information is available on xylometazoline pharmacokinetics. Metabolism / Metabolites No information is available on xylometazoline pharmacokinetics. Biological Half-Life No information is available on xylometazoline pharmacokinetics. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
No information is available on xylometazoline pharmacokinetics. |
||
| 参考文献 | |||
| 其他信息 |
Xylometazoline is an alkylbenzene.
Xylometazoline is an imidazoline derivative with sympathomimetic and nasal decongestant activity. Xylometazoline works by binding to alpha (α)-adrenergic receptors to cause vasoconstriction of nasal blood vessels. Xylometazoline is available in over-the-counter (OTC) nasal sprays or drops to temporarily relieve nasal congestion due to cold, hay fever or other respiratory allergies. In some countries, it is available as combination products with [ipratropium], [domiphen], or [dexpanthenol]. See also: Xylometazoline Hydrochloride (has salt form). Drug Indication Xylometazoline is indicated for the temporary relief of nasal congestion due to cold, hay fever or other respiratory allergies. Mechanism of Action Nasal congestion is caused by various etiologies, such as rhinosinusitis and allergic or non-allergic rhinitis, leading to congestion of the venous sinusoids lining the nasal mucosa. Activation of α-adrenergic receptors leads to vasoconstriction of the blood vessels of the nasal mucosa and resumption of nasal airflow. As the most abundantly expressed in the human nasal mucosa, α1A- and α2B-adrenoceptors may play the most important role in vasoconstriction of the human nasal mucosa. Xylometazoline is a more selective agonist at α2B-adrenoceptors, with affinity at α1A-, α2A-, α2C-, α1B-, and α1D-adrenoceptors. Xylometazoline decreases nasal resistance during inspiration and expiration and increases the volume of nasal airflow. Compared to [oxymetazoline], another imidazoline nasal decongestant, xylometazoline had a slightly faster onset of action although they had a similar duration of action. In one study, subjects with nasal congestion reported relief of earache and sore throat in addition to nasal decongestion: it is speculated that oxymetazoline mediates this effect by causing vasoconstriction of the nasal mucosa that contains the venous sinuses and nasal decongestion allows breathing through the nose, providing relief from sore throat caused by mouth breathing that dries and irritates the throat. Pharmacodynamics Xylometazoline is a sympathomimetic agent that causes vasoconstriction of the nasal mucosa. In one study comprising subjects with nasal congestion associated with the common cold, the median time of onset of subjective relief of nasal congestion was about 1.7 minutes and the time of subjective peak relief of nasal congestion was 30 minutes. Previous studies reported rebound swelling, rebound nasal congestion, rhinitis medicamentosa, and shorter duration of decongestant effect from the long-term use of xylometazoline in healthy volunteers, suggesting that the drug is most effective if used temporarily. An early _in vitro_ study demonstrated xylometazoline to exert anti-oxidant actions, where it inhibited microsomal lipid peroxidation and mediated hydroxyl radical scavenging activity. This suggests that xylometazoline has a beneficial effect against oxidants, which play a role in tissue damage in inflammation. |
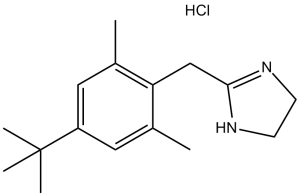
| 分子式 |
C16H25CLN2
|
|---|---|
| 分子量 |
280.84
|
| 精确质量 |
280.17
|
| 元素分析 |
C, 68.43; H, 8.97; Cl, 12.62; N, 9.98
|
| CAS号 |
1218-35-5
|
| 相关CAS号 |
Xylometazoline; 526-36-3
|
| PubChem CID |
5709
|
| 外观&性状 |
White to off-white crystalline powder
|
| 沸点 |
394.2ºC at 760 mmHg
|
| 熔点 |
131-133ºC
|
| 闪点 |
192.2ºC
|
| 蒸汽压 |
4.56E-06mmHg at 25°C
|
| LogP |
3.711
|
| tPSA |
24.39
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
18
|
| 分子复杂度/Complexity |
302
|
| 定义原子立体中心数目 |
0
|
| SMILES |
Cl[H].N1([H])C([H])([H])C([H])([H])N=C1C([H])([H])C1C(C([H])([H])[H])=C([H])C(=C([H])C=1C([H])([H])[H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H]
|
| InChi Key |
YGWFCQYETHJKNX-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C16H24N2.ClH/c1-11-8-13(16(3,4)5)9-12(2)14(11)10-15-17-6-7-18-15;/h8-9H,6-7,10H2,1-5H3,(H,17,18);1H
|
| 化学名 |
2-[(4-tert-butyl-2,6-dimethylphenyl)methyl]-4,5-dihydro-1H-imidazole;hydrochloride
|
| 别名 |
xylomethazoline; Xylometazoline HCl; Brand name: Decongest; Otrivine; Balkis; Amidrin; Chlorohist-LA; espa-rhin; Gelonasal
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.90 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.90 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (8.90 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 16.67 mg/mL (59.36 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.5607 mL | 17.8037 mL | 35.6075 mL | |
| 5 mM | 0.7121 mL | 3.5607 mL | 7.1215 mL | |
| 10 mM | 0.3561 mL | 1.7804 mL | 3.5607 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05334017 | Completed | Drug: Xylometazoline 0,1% Drug: Cocaine 4% |
Epistaxis | Rigshospitalet, Denmark | September 8, 2022 | Phase 4 |
| NCT03424889 | Completed | Drug: Xylometazoline Drug: Saline |
Bronchoscopy | All India Institute of Medical Sciences, New Delhi |
June 1, 2018 | Not Applicable |
| NCT00452270 | Completed | Drug: Xylometazoline | Common Cold | Novartis | March 2007 | Phase 3 |
| NCT00622817 | Completed | Drug: xylometazoline HCL 0.05% Drug: Epinephrine 1mg |
Bronchiolitis | Schneider Children's Medical Center, Israel |
October 2004 | Not Applicable |