| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
leukotriene receptor/LTD4
|
|---|---|
| 体外研究 (In Vitro) |
扎鲁司特具有 N-甲基吲哚部分,吲哚环上有 3-烷基取代基,这使其与 3-甲基吲哚相似。与辣根过氧化物酶一起孵育后,扎鲁司特很容易形成分子量为 880 Da 的 GSH 加合物(60 分钟内转化率为 10%)。扎鲁司特以浓度、时间和 NADP(H) 依赖性方式灭活 CYP3A4。在一份报告中,扎鲁司特被证明可以在体外抑制 CYP3A 酶活性(非预孵育抑制),Ki 为 2 μM;然而,已经公布了更高的值,我们的灭活过程 KI 为 13.4 μM。扎鲁司特的高血浆蛋白结合率(>99%)会降低游离药物的浓度。扎鲁司特连续两次单电子氧化产生高度亲电子的 α,β-不饱和亚胺物种,然后自发与 GSH 反应产生观察到的加合物。 [1]
|
| 体内研究 (In Vivo) |
Zafirlukast 是一种肽基白三烯拮抗剂和 LTD4 。 暴露 13 周后,两种剂量水平的 Zafirlukast (270 和 540 mg/kg)、高剂量 Zafirlukast (1200 mg/kg) 和含有 600 mg/ kg 齐留通的组合与扎鲁司特或 MK-866 一起显着降低了肺的产量。含有齐留通和扎鲁司特的组合预防肿瘤的功效与单独使用三次肺癌的功效没有显着差异。虽然当联合用药时的剂量单独浓度时,Zileuton 或 MK-886 都不能预防肿瘤;包含它们的组合确实可以显着着预防肿瘤。相比之下,包含 Zafirlukast 和 MK-886 的组合不会降低肿瘤的产量,而单独使用扎鲁司特会显着降低肿瘤产量[2]。
|
| 酶活实验 |
扎鲁司特是一种白三烯拮抗剂,用于治疗轻中度哮喘,但该药物偶尔会出现特异性肝毒性。从结构上讲,扎鲁司特与3-甲基吲哚相似,因为它含有一个N-甲基吲哚部分,该部分在吲哚环上有一个3-烷基取代基。这里的结果描述了扎鲁司特通过类似于3-甲基吲哚的机制进行的代谢活化。大鼠和人类肝微粒体对扎鲁司特的NADP(H)依赖性生物转化提供了一种反应性代谢产物,被检测为其GSH加合物。质谱和核磁共振数据表明,GSH加合物是通过将GSH加成扎鲁司特吲哚和甲氧基取代苯环之间的亚甲基碳而形成的。这种反应性代谢物在人肝微粒体中的形成被证明仅由CYP3A酶催化。当静脉注射或口服扎鲁司特的大鼠胆汁中检测到相同的GSH加合物时,获得了扎鲁司t体内代谢激活的证据。根据模型过氧化物酶的结果和含H2(18)O孵育产物醇的结构,扎鲁司特似乎经历了两次连续的单电子氧化脱氢。此外,扎鲁司特被证明是人肝微粒体和含有表达CYP3A4的cDNA的微粒体中CYP3A4活性的基于机制的抑制剂。在人类肝微粒体中测试的所有P450酶中,扎鲁司特的酶抑制特性对这种酶具有选择性。失活的特征是K(I)为13.4微米,K(inact)为0.026分钟(-1)。总之,扎鲁司特脱氢为亲电α,β不饱和亚胺中间体可能与特异性肝毒性有关,和/或通过CYP3A4失活引起药物相互作用[1]
|
| 细胞实验 |
1.我们研究了半胱氨酰白三烯(CysLT1)受体拮抗剂普仑司特和扎鲁司特对豚鼠气管中由白三烯D4(LTD4)或致敏动物抗原攻击诱导的35SO4标记粘液输出的抑制作用。激动剂和拮抗剂通过粘膜给药,但在选定的比较实验中除外,在这些实验中,药物通过粘膜和血清给药来评估上皮对诱发分泌的影响。2.LTD4以浓度相关的方式增加了35SO4的产量,在100微米时比对照组增加了23倍,EC50约为2微米。与单独添加粘膜相比,LTD4的粘膜和浆膜联合添加对分泌反应没有显著影响。LTC4和LTE4(各10微米)均未影响35SO4的输出。Pranlukast或zafirlukast以浓度依赖的方式显著抑制了10微M LTD4诱发的35SO4输出,10微M Pranlukast和10微M zafirlukat的最大抑制率分别为83%和78%,pranlukat和zafirlucast的IC50值分别为0.3微M和0.6微M。拮抗剂的粘膜和浆膜联合给药(每种5微M)对粘膜-浆膜的抑制程度为10微M,LTD4诱发的35SO4输出与粘膜给药相似。Pranlukast(0.5微M)导致LTD4浓度响应曲线平行向右移动,pKB为7。Pranlukast不抑制ATP诱导的35SO4输出。3.卵清蛋白(10-500微克/毫升(-1))对用卵清蛋白主动致敏的豚鼠气管的攻击导致35SO4输出的浓度相关增加,在200微克/毫升时,最大增加量比载体对照组高20倍(-1)。抗组胺药吡拉明和西咪替丁(各0.1mM)的组合没有抑制致敏豚鼠卵清蛋白诱导的35SO4输出。粘膜(10微米或100微米)和粘膜浆膜(100微米)组胺对35SO4的输出都没有显著影响。4.普仑司特或扎鲁司特(各5μM)分别显著抑制了致敏豚鼠气管中卵清蛋白诱导的分泌,抑制率分别为70%和65%。5我们得出结论,致敏动物的LTD4或卵清蛋白攻击在体外会引起豚鼠气管粘液分泌,这种作用受到CysLT1受体拮抗剂普仑司特和扎鲁司特的抑制。这些拮抗剂可能有益于治疗粘液高分泌是临床症状的过敏性气道疾病,例如哮喘和过敏性鼻炎[3]。
|
| 动物实验 |
Mice: It uses A/J female mice that are 5–6 weeks old. The first of two intraperitoneal injections (i.p.) of vinyl carbamate (16 mg/kg each, spaced seven days apart) is given to the mice when they are seven to eight weeks old. In their diet, the mice are given leukotriene inhibitors two weeks following their second dosage of vinyl carbamate. The meal should contain the prescribed mg/kg concentrations of zafirlukast (270 or 540 mg/kg), zileuton (600 or 1200 mg/kg), and MK-886 (30 mg/kg). After being exposed to leukotriene inhibitors for the first six weeks, mice are weighed every week. They are weighed every two to four weeks until they are sacrificed after that. Before being embedded in paraffin for histology, the lungs are removed, fixed for one night in formalin, moved to 70% alcohol, and checked for tumors.
The present work was conducted to assess the possible protective effects of zafirlukast against the toxic damage induced by acetic acid in rat colon. Zafirlukast is a potent and selective cysteinyl leukotriene receptor antagonist which is used mainly in the prophylaxis of bronchial asthma. Two doses of zafirlukast were used (40 and 80 mg/kg) dissolved in gum acacia and given either orally or rectally (0.5 ml/kg). Several parameters including, macroscopic score, histopathological and biochemical such as malondialdehyde (MDA), myeloperoxidase (MPO), catalase and reduced glutathione (GSH) levels were measured using standard assay procedures. The study showed that pretreatment with zafirlukast in a dose of 80 mg/kg orally produced a significant decrease in tissue malondialdehyde, myeloperoxidase, and an increase in both reduced glutathione and catalase levels, while there was no significant changes with the rectal route. The 40 mg/kg dose had no significant protective effects when given either orally or rectally. The available data indicate that the inhibition of leukotriene synthesis or action may have a role in inflammatory bowel disease (IBD) as they are considered as important mediators in this condition.[3] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Rapidly absorbed following oral administration, reduced following a high-fat or high-protein meal. The most common metabolic products are hydroxylated metabolites which are excreted in the feces. 70 L apparent oral CL=20 L/h 11.4 L/h [7-11 yrs] 9.2 L/h [5-6 yrs] Metabolism / Metabolites Hepatic Zafirlukast has known human metabolites that include cyclopentyl N-[1-(hydroxymethyl)-3-({2-methoxy-4-[(2-methylbenzenesulfonyl)carbamoyl]phenyl}methyl)indol-5-yl]carbamate and 3-hydroxycyclopentyl N-[3-({2-methoxy-4-[(2-methylbenzenesulfonyl)carbamoyl]phenyl}methyl)-1-methylindol-5-yl]carbamate. Biological Half-Life 10 hours |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Prospective studies have shown that ALT elevations occur in 1.5% of patients receiving zafirlukast, most of which are mild, asymptomatic and self limited even with continuing therapy. A similar rate of transient ALT elevations is often found in placebo recipients. Clinically apparent liver disease from zafirlukast is rare, but many convincing cases have been reported, several of which were severe and resulted in hepatic failure, need for liver transplantation or death. The onset of symptoms of liver injury is typically within 2 to 6 months of starting therapy, but cases with longer latency periods have been reported (8-13 months). Symptoms include fatigue, nausea, and right upper quadrant pain followed by dark urine, jaundice and pruritus. The pattern of liver enzyme elevation is usually hepatocellular and resembles acute viral hepatitis. Eosinophilia is common but immunoallergic features are usually not prominent and autoantibodies are uncommon (eosinophilia may be due to the underlying allergic asthmatic condition). Fatal cases have been described. Reexposure can lead to more rapid and severe reappearance of injury and should be avoided. Zafirlukast can also cause systemic vasculitis and eosinophilia (Churg-Strauss Syndrome) which may be accompanied by hepatic involvement and mild enzyme elevations. Likelihood score: C (probable cause of clinical apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No published information is available on the use of zafirlukast during breastfeeding; however, manufacturer's data indicate that the dose in milk is low. Zafirlukast has been used in children as young as 12 months of age. International guidelines consider that leukotriene receptor antagonists can be used during breastfeeding. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding 99% |
| 参考文献 | |
| 其他信息 |
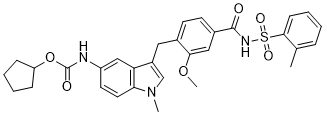
Zafirlukast is a member of indoles, a carbamate ester and a N-sulfonylcarboxamide. It has a role as an anti-asthmatic agent and a leukotriene antagonist.
Zafirlukast is an oral leukotriene receptor antagonist (LTRA) for the maintenance treatment of asthma, often used in conjunction with an inhaled steroid and/or long-acting bronchodilator. It is available as a tablet and is usually dosed twice daily. Another leukotriene receptor antagonist is montelukast (Singulair), which is usually taken just once daily. Zafirlukast blocks the action of the cysteinyl leukotrienes on the CysLT1 receptors, thus reducing constriction of the airways, build-up of mucus in the lungs and inflammation of the breathing passages. Zafirlukast is a Leukotriene Receptor Antagonist. The mechanism of action of zafirlukast is as a Leukotriene Receptor Antagonist, and Cytochrome P450 2C9 Inhibitor. Zafirlukast is an orally available leukotriene receptor antagonist which is widely used for the prophylaxis and chronic treatment of asthma. Zafirlukast has been linked to rare, but occasionally severe cases of acute liver injury. Zafirlukast is a tolyl compound and leukotriene receptor antagonist (LTRA), with anti-asthmatic and potential capsular contracture-preventing activities. Upon administration, zafirlukast selectively and competitively binds to and blocks the cysteinyl leukotriene 1 receptor (CYSLTR1), thereby preventing the potent pro-inflammatory mediators leukotriene C4, D4 and E4 from binding. This prevents leukotriene-mediated actions, including enhanced migration of eosinophils and neutrophils, increased adhesion of leukocytes, increased monocyte and neutrophil aggregation, increased airway edema, inflammation, capillary permeability and bronchoconstriction. In addition, zafirlukast may decrease collagen deposition, fibrosis, and capsular thickness after implantation, thereby preventing scar tissue. Drug Indication For the prophylaxis and chronic treatment of asthma. FDA Label Mechanism of Action Zafirlukast is a selective and competitive receptor antagonist of leukotriene D4 and E4 (LTD4 and LTE4), components of slow-reacting substance of anaphylaxis (SRSA). Cysteinyl leukotriene production and receptor occupation have been correlated with the pathophysiology of asthma, including airway edema, smooth muscle constriction, and altered cellular activity associated with the inflammatory process, which contribute to the signs and symptoms of asthma. Pharmacodynamics Zafirlukast is a synthetic, selective peptide leukotriene receptor antagonist (LTRA) indicated for the prophylaxis and chronic treatment of asthma. Patients with asthma were found in one study to be 25-100 times more sensitive to the bronchoconstricting activity of inhaled LTD4 than nonasthmatic subjects. In vitro studies demonstrated that zafirlukast antagonized the contractile activity of three leukotrienes (LTC4, LTD4 and LTE4) in conducting airway smooth muscle from laboratory animals and humans. Zafirlukast prevented intradermal LTD4-induced increases in cutaneous vascular permeability and inhibited inhaled LTD4-induced influx of eosinophils into animal lungs. |
| 分子式 |
C31H33N3O6S
|
|---|---|
| 分子量 |
575.6752
|
| 精确质量 |
575.208
|
| 元素分析 |
C, 64.68; H, 5.78; N, 7.30; O, 16.67; S, 5.57
|
| CAS号 |
107753-78-6
|
| 相关CAS号 |
Zafirlukast-d7; 1217174-18-9; Zafirlukast-13C,d3; Zafirlukast-13C,d6
|
| PubChem CID |
5717
|
| 外观&性状 |
Off-white to pink solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 熔点 |
139°C
|
| 折射率 |
1.641
|
| LogP |
6.15
|
| tPSA |
131.09
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
41
|
| 分子复杂度/Complexity |
1010
|
| 定义原子立体中心数目 |
0
|
| SMILES |
S(C1=C([H])C([H])=C([H])C([H])=C1C([H])([H])[H])(N([H])C(C1C([H])=C([H])C(=C(C=1[H])OC([H])([H])[H])C([H])([H])C1=C([H])N(C([H])([H])[H])C2C([H])=C([H])C(=C([H])C1=2)N([H])C(=O)OC1([H])C([H])([H])C([H])([H])C([H])([H])C1([H])[H])=O)(=O)=O
|
| InChi Key |
YEEZWCHGZNKEEK-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35)
|
| 化学名 |
cyclopentyl N-[3-[[2-methoxy-4-[(2-methylphenyl)sulfonylcarbamoyl]phenyl]methyl]-1-methylindol-5-yl]carbamate
|
| 别名 |
ICI-204219; ICI204219; ICI 204219; ICI-204,219; ICI 204,219; ICI204,219; Zafirlukast; brand names Accolate, Accoleit, and Vanticon; Olmoran; Cyclopentyl (3-(2-methoxy-4-((o-tolylsulfonyl)carbamoyl)benzyl)-1-methyl-1H-indol-5-yl)carbamate;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~100 mg/mL (~173.7 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (3.61 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (3.61 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 5%DMSO + 40%PEG300 + 5%Tween 80 + 50%ddH2O: 5.0mg/ml (8.69mM) 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7371 mL | 8.6854 mL | 17.3708 mL | |
| 5 mM | 0.3474 mL | 1.7371 mL | 3.4742 mL | |
| 10 mM | 0.1737 mL | 0.8685 mL | 1.7371 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04339140 | Recruiting | Drug: Zafirlukast | Ovarian Cancer | Beth Israel Deaconess Medical Center |
June 24, 2020 | Phase 2 |
| NCT06069063 | Not yet recruiting | Drug: Zafirlukast | Allergy to Cats | Allergy & Asthma Medical Group & Research Center |
January 5, 2024 | Phase 2 |
| NCT01283061 | Completed | Drug: Zafirlukast | Healthy | Dr. Reddy's Laboratories Limited | December 2007 | Phase 1 |
| NCT02950480 | Terminated | Drug: zafirlukast Other: Standard of Care (no intervention) |
Breast Cancer | University of California, San Francisco |
March 13, 2017 | Phase 2 |