| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
| 靶点 |
αvβ6; Integrins αvβ6 (KD = 5.7 nM) and αvβ1 (KD = 3.4 nM)
|
|---|---|
| 体外研究 (In Vitro) |
在精密肺切片 (PCLS) 中,贝索格斯特(PLN-74809;1.82 µM;肠道 7 天)显着降低 I 型胶原 (COL1A1) mRNA 表达 54%。贝索司特中的 Smad2 磷酸化较少。在由纤维化肺产生的 PCLS 中,贝索格拉斯特(1.82 µM;工作站 3 天)可自我调节,使 Col1a1 mRNA 表达降低高达 71% [2]。 αvβ6 整合素被贝索格斯特完全阻断。大约50%[2]。 LAP 由正常人上皮细胞标记,IC50 为 39.3 nM[2]。
抑制TGF - β激活:Bexotegrast (PLN - 74809)可通过抑制整合素αvβ6和αvβ1,阻断纤维化肺细胞中的多种TGF - β激活途径,通过抑制TGF - β的激活来减少I型胶原基因的表达[1] |
| 体内研究 (In Vivo) |
Bexotegrast(PLN-74809;侧壁;100、250、500 mg/kg;每天两次;从第 7 天到第 21 天)以抑制博莱霉素攻击的软骨间质纤维胶原沉积所需的水平提供。
抗纤维化作用:在博来霉素处理的小鼠中,Bexotegrast (PLN - 74809)可剂量依赖性地抑制肺部Smad3磷酸化和胶原蛋白沉积,减轻肺纤维化负担。其抗纤维化效果优于尼达尼布或吡非尼酮,能更有效地降低纤维化小鼠肺组织中的胶原基因表达[2] |
| 动物实验 |
Animal/Disease Models: C57BL/6 mice [2]
Doses: 100, 250, 500 mg/kg Route of Administration: Oral; strongly bursts Smad3 phosphorylation [2]. twice (two times) daily; from day 7 to day 21 Experimental Results: There was a dose-dependent significant reduction in interstitial fibrillar collagen deposition in mice challenged with bleomycin (3 units/kg). Dose-dependent blockade of Smad3 phosphorylation. Bleomycin - induced lung fibrosis mouse model: Use bleomycin to induce lung fibrosis in mice. Then, administer Bexotegrast (PLN - 74809) to the mice, and set up different dose - groups. After a certain period of treatment, sacrifice the mice, collect lung tissues, and detect the changes of pulmonary collagen deposition and Smad3 phosphorylation, so as to evaluate the antifibrotic effect of the drug [2] |
| 药代性质 (ADME/PK) |
Pharmacokinetics [Am J Respir Crit Care Med
. 2024 Aug 15;210(4):424-434.]
Predicted total and unbound exposures (maximum concentration and area under the concentration time curve from Time 0 to 24 hours postdose) of bexotegrast in participants with IPF increased approximately proportionally with dose (see Tables E3 and E4). |
| 毒性/毒理 (Toxicokinetics/TK) |
Safety and Tolerability through Week 12 [Am J Respir Crit Care Med
. 2024 Aug 15;210(4):424-434.]
Overall, bexotegrast demonstrated a favorable safety and tolerability profile over 12 weeks of treatment (Tables 2, 3, E1, and E2 in the online supplement). The majority of TEAEs were mild or moderate in severity. The most common TEAEs are presented in Table 2. Diarrhea was the most common TEAE, occurring in 15 participants (16.9%) in the bexotegrast (pooled) group and 3 participants (9.7%) in the placebo group. Of the participants reporting diarrhea, 13/15 (86.7%) participants in the bexotegrast group and 1/3 (33.3%) participants in the placebo group were receiving nintedanib background therapy; one participant (6.7%) with diarrhea in the bexotegrast group was receiving pirfenidone (Table 3). The remaining participant with diarrhea who received bexotegrast monotherapy had preexisting ulcerative colitis. Most events of diarrhea (14/15; 93.3%) were mild to moderate in severity; one participant receiving bexotegrast and pirfenidone had Grade 3 diarrhea. Four participants interrupted bexotegrast treatment because of intercurrent coronavirus disease (COVID-19) (n = 1), diarrhea (n = 2), and ileus and acute kidney injury (n = 1). Two participants discontinued bexotegrast because of mild diarrhea (n = 1 receiving background nintedanib; n = 1 receiving no background therapy and having preexisting ulcerative colitis). Dose reduction of bexotegrast was not allowed per protocol. No serious adverse events were assessed as being related to the study drug. TEAEs of IPF/pulmonary fibrosis were reported in 2.3% of participants in the bexotegrast group and 9.7% of participants in the placebo group. Only one of these events was reported as an acute exacerbation of IPF in a participant who had completed 12 weeks of treatment with 160 mg bexotegrast and experienced the event 11 days after the last dose. The event was not considered to be drug related and resolved the following day after treatment in hospital with corticosteroids and antibiotics. One participant with gender-age-physiology Stage III IPF and preexisting coronary artery disease and chronic refractory atrial fibrillation in the 320-mg bexotegrast group experienced a serious and fatal adverse event of acute respiratory failure after elective atrioventricular node ablation. There were no notable changes in laboratory parameters, vital signs, physical examination findings, or electrocardiography findings associated with the study drug. Overall, bexotegrast was well tolerated when used in combination with IPF background therapy or when used as monotherapy (Tables 3, E1, and E2). |
| 参考文献 | |
| 其他信息 |
αv integrins are key regulators of TGF - β activation and fibrogenesis in pulmonary fibrosis models. αvβ6 and αvβ1 are highly expressed in the lung tissue of patients with idiopathic pulmonary fibrosis, while they are rarely expressed in normal tissues. Bexotegrast (PLN - 74809) is a small - molecule oral drug, which can block the activation of TGF - β by αvβ6 and αvβ1, and may slow down or even stop the fibrosis process in patients with idiopathic pulmonary fibrosis. The phase Ⅱ/Ⅲ clinical trial of treating IPF is in progress [2]
PLN-74809 is a small-molecule that dually inhibits both αvβ6 and αvβ1 to treat both idiopathic pulmonary fibrosis (IPF) and primary sclerosing cholangitis (PSC). The compound was granted orphan drug designation by the US FDA in August 2018 for treatment of IPF, and Pliant Therapeutics recently raised $100 million in Series C financing to support further clinical development of this compound. Phase 2a studies are currently evaluating PLN-74809 safety and efficacy for participants with IPF (NCT04396756)across 10 participating countries, and for participants with PSC (NCT04480840). In addition, the compound is also currently in Phase 2a trials (NCT04565249) for the treatment of COVID-19 related acute respiratory distress syndrome. Drug Indication Through dual inhibition of integrins αvβ6 and αvβ1, PLN-74809 reduces subsequent activation of TGF-β1, which is actively involved in the growth of fibrotic tissue in lung and bile ducts. Further studies showed that PLN-74809 inhibited collagen gene expression in PSC and IPF patient tissue. |
| 分子式 |
C27H36N6O3
|
|---|---|
| 分子量 |
492.61
|
| 精确质量 |
492.28
|
| 元素分析 |
C, 65.83; H, 7.37; N, 17.06; O, 9.74
|
| CAS号 |
2376257-44-0
|
| 相关CAS号 |
2376264-69-4 (R-isomer); 2376257-44-0;2775365-40-5 (HCl);2775365-33-6 (fumarate); 2775365-31-4 (phosphate);
|
| PubChem CID |
135390719
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
1.8
|
| tPSA |
113
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
14
|
| 重原子数目 |
36
|
| 分子复杂度/Complexity |
655
|
| 定义原子立体中心数目 |
1
|
| SMILES |
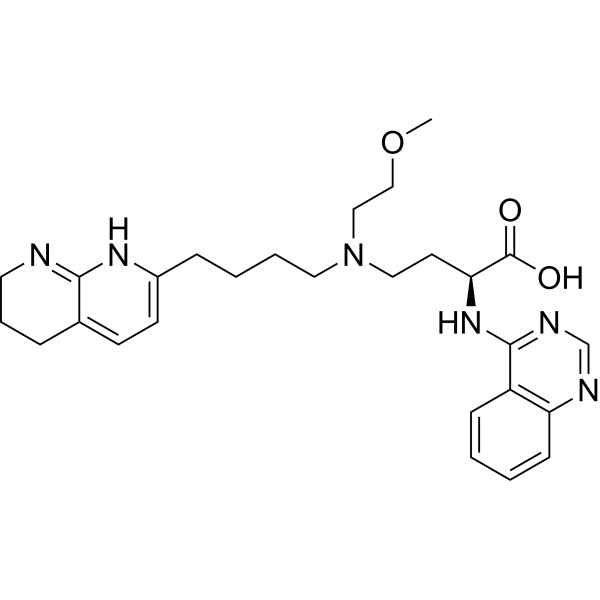
COCCN(CCCCC1=NC2=C(CCCN2)C=C1)CC[C@@H](C(=O)O)NC3=NC=NC4=CC=CC=C43
|
| InChi Key |
CWOFQJBATWQSHL-DEOSSOPVSA-N
|
| InChi Code |
InChI=1S/C27H36N6O3/c1-36-18-17-33(15-5-4-8-21-12-11-20-7-6-14-28-25(20)31-21)16-13-24(27(34)35)32-26-22-9-2-3-10-23(22)29-19-30-26/h2-3,9-12,19,24H,4-8,13-18H2,1H3,(H,28,31)(H,34,35)(H,29,30,32)/t24-/m0/s1
|
| 化学名 |
(S)-4-((2-methoxyethyl)(4-(5,6,7,8-tetrahydro-1,8-naphthyridin-2-yl)butyl)amino)-2-(quinazolin-4-ylamino)butanoic acid
|
| 别名 |
PLN-74809; PLN 74809; PLN-74,809; Bexotegras; Bexotegras [INN]; (S)-4-((2-Methoxyethyl)(4-(5,6,7,8-tetrahydro-1,8-naphthyridin-2-yl)butyl)amino)-2-(quinazolin-4-ylamino)butanoic acid; (S)-4-[(2-Methoxyethyl)[4-(5,6,7,8-tetrahydro-1,8-naphthyridin-2-yl)butyl]amino]-2-(quinazolin-4-ylamino)butanoic Acid; Bexotegrast; 2376257-44-0; QCV154PFT4; PLN74809; Bexotegrast; Bexotegrast free base,
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 250 mg/mL (507.50 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.22 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.22 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.22 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0300 mL | 10.1500 mL | 20.3000 mL | |
| 5 mM | 0.4060 mL | 2.0300 mL | 4.0600 mL | |
| 10 mM | 0.2030 mL | 1.0150 mL | 2.0300 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。