| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
| 靶点 |
D2 dopamine receptors; 5-HT2A[1][6].
|
|---|---|
| 体外研究 (In Vitro) |
Chlorpromazine 氯丙嗪(0、10、20、40 μM;0、24、48 小时)以时间和剂量依赖性方式抑制 U-87MG 胶质瘤细胞增殖[2]。 12 小时后,氯丙嗪(20 μM;0、12、24、48 小时)可降低 U-87MG 胶质瘤细胞中细胞周期蛋白 A 和细胞周期蛋白 B1 的水平[2]。 20 μM 氯丙嗪可抑制细胞周期进程[2]。在sEV处理的骨髓细胞中,氯丙嗪(10 μM;1 h)显着降低MDSC并显着抑制sEV的内化(MDSC可以显着抑制免疫细胞反应,在癌细胞中产生免疫抑制)[3]。 hNav1.7 电流被氯丙嗪(3、10、20、40 和 60 µM)以浓度依赖性方式阻断[4]。 HERG 钾通道被氯丙嗪阻断,其 IC50 值为 21.6 µM,希尔系数为 1.11[5]。
|
| 体内研究 (In Vivo) |
氯丙嗪(20 mg/kg;腹腔注射;每天一次,持续 7 天)抑制裸鼠异种移植肿瘤的生长[2]。
|
| 细胞实验 |
细胞增殖测定[2]
细胞类型: U-87MG 胶质瘤细胞 测试浓度: 0、10、20、40 μM 孵育时间:0、24、48 小时 实验结果:以剂量和时间依赖性方式证明抗增殖活性。 免疫荧光[3] 细胞类型: 骨髓细胞(经 sEV 处理) 测试浓度: 10 µM 孵育时间:1小时 实验结果:减少MDSC并抑制sEV内化。 蛋白质印迹分析[2] 细胞类型: U-87MG 神经胶质瘤细胞 测试浓度: 20 μM 孵育时间:0、12、24、48小时 实验结果:12小时后细胞周期蛋白A和细胞周期蛋白B1的水平降低,而增殖细胞的细胞周期蛋白D1的水平降低核抗原和GAPDH保持不变。 |
| 动物实验 |
Animal/Disease Models: 5- to 6 weeks old athymic nude mice bearing intracranial U-87MG xenograft tumors[2].
Doses: 20 mg/kg Route of Administration: Injected intraperitoneally (ip); single daily for 7 days Experimental Results: Inhibited tumor growth on day 17. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Readily absorbed from the GI tract. Bioavailability varies due to first-pass metabolism by the liver. Kidneys, ~ 37% excreted in urine 20 L/kg Chlorpromazine hydrochloride is rapidly absorbed from the GI tract and from parenteral sites of injection; however, following oral administration, the drug undergoes considerable metabolism during absorption (in the GI mucosa) and first pass through the liver. Although not clearly established in humans, chlorpromazine and its metabolites undergo enterohepatic circulation in animals. Considerable interindividual variations in peak plasma concentrations have been reported with the same oral dose of chlorpromazine. The variability is thought to result from wide interindividual variation in bioavailability, apparently because of genetic differences in the rate of first-pass metabolism. As a result of first-pass metabolism, less chlorpromazine reaches systemic circulation as unchanged drug, and peak plasma chlorpromazine concentrations are much lower following oral administration than following im administration. Following oral administration of chlorpromazine hydrochloride in a tablet formulation, the onset of pharmacologic action occurs within 30-60 minutes; the duration of action is 4-6 hours. The onset of pharmacologic action following oral administration of chlorpromazine hydrochloride in an extended-release formulation is approximately 30-60 minutes; the duration of action is 10-12 hours. The onset of pharmacologic action following rectal administration of chlorpromazine is generally slower than that following oral administration of chlorpromazine hydrochloride; rectally administered chlorpromazine has a duration of action of 3-4 hours. Phenothiazines and their metabolites are distributed into most body tissues and fluids, with high concentrations being distributed into the brain, lungs, liver, kidneys, and spleen. /Phenothiazine General Statement/ For more Absorption, Distribution and Excretion (Complete) data for Chlorpromazine (17 total), please visit the HSDB record page. Metabolism / Metabolites Extensively metabolized in the liver and kidneys. It is extensively metabolized by cytochrome P450 isozymes CYP2D6 (major pathway), CYP1A2 and CYP3A4. Approximately 10 to 12 major metabolite have been identified. Hydroxylation at positions 3 and 7 of the phenothiazine nucleus and the N-dimethylaminopropyl side chain undergoes demethylation and is also metabolized to an N-oxide. In urine, 20% of chlopromazine and its metabolites are excreted unconjugated in the urine as unchanged drug, demonomethylchlorpromazine, dedimethylchlorpromazine, their sulfoxide metabolites, and chlorpromazine-N-oxide. The remaining 80% consists of conjugated metabolites, principally O-glucuronides and small amounts of ethereal sulfates of the mono- and dihydroxy-derivatives of chlorpromazine and their sulfoxide metabolites. The major metabolites are the monoglucuronide of N-dedimethylchlorpromazine and 7-hydroxychlorpromazine. Approximately 37% of the administered dose of chlorpromazine is excreted in urine. Although the exact metabolic fate of chlorpromazine is not clearly established, the drug is extensively metabolized, principally in the liver and kidneys. About 10-12 metabolites which occur in humans in appreciable quantities have been identified. In addition to hydroxylation at positions 3 and 7 of the phenothiazine nucleus, the N-dimethylaminopropyl side chain of chlorpromazine undergoes demethylation and is also metabolized to an N-oxide. Two principal groups of metabolites have been found in urine. The unconjugated fraction, which represents approximately 20% of chlorpromazine and its metabolites excreted in urine, consists of unchanged drug, demonomethylchlorpromazine, dedimethylchlorpromazine, their sulfoxide metabolites, and chlorpromazine-N-oxide. The conjugated fraction, which represents approximately 80% of chlorpromazine and its metabolites excreted in urine, consists principally of O-glucuronides, with small amounts of ethereal sulfates of the mono- and dihydroxy-derivatives of chlorpromazine and their sulfoxide metabolites. The major metabolites found in urine are the monoglucuronide of N-dedimethylchlorpromazine and 7-hydroxychlorpromazine. Most metabolites of phenothiazines are pharmacologically inactive; however, certain metabolites (eg, 7-hydroxychlorpromazine, mesoridazine) show moderate pharmacologic activity and may contribute to the action of the drugs. There is limited evidence to indicate that some phenothiazines (eg, chlorpromazine) may induce their own metabolism. /Phenothiazine General Statement/ Yields 2-chlorophenothiazine in man; promazine probably in dog and in man ... . Yields demethylchlorpromazine in man, rat, rabbit, mouse, dog, sheep, guinea pig ... . Yields chlorpromazine sulfoxide in man, rat, rabbit, dog ... . Yields chlorpromazine-n-oxide in man, rat, rabbit, dog, pig, sheep, guinea pig, mouse ... . Yields 3-hydroxychlorpromazine in man, rat, dog ... . Yields 7-hydroxychlorpromazine in man, rat, sheep, dog, rabbit, guinea pig ... . /From table/ As least 10 or 12 metabolites of chlorpromazine occur in human beings in appreciable quantities. Quantitatively, the most important of these are nor2-chlorpromazine (doubly methylated), chlorphenothiazine (removal of the entire side chain), methoxy and hydroxy products, and glucuronide conjugates of the hydroxylated compounds. In urine, 7-hydroxylated and dealkylated (nor2) metabolites and their conjugates predominate. Chlorpromazine has known human metabolites that include 7-Hydroxychlorpromazine, Chlorpromazine S-oxide, Chlorpromazine, N-desmethyl, and Chlorpromazine N-glucuronide. Extensively metabolized in the liver and kidneys. It is extensively metabolized by cytochrome P450 isozymes CYP2D6 (major pathway), CYP1A2 and CYP3A4. Approximately 10 to 12 major metabolite have been identified. Hydroxylation at positions 3 and 7 of the phenothiazine nucleus and the N-dimethylaminopropyl side chain undergoes demethylation and is also metabolized to an N-oxide. In urine, 20% of chlopromazine and its metabolites are excreted unconjugated in the urine as unchanged drug, demonomethylchlorpromazine, dedimethylchlorpromazine, their sulfoxide metabolites, and chlorpromazine-N-oxide. The remaining 80% consists of conjugated metabolites, principally O-glucuronides and small amounts of ethereal sulfates of the mono- and dihydroxy-derivatives of chlorpromazine and their sulfoxide metabolites. The major metabolites are the monoglucuronide of N-dedimethylchlorpromazine and 7-hydroxychlorpromazine. Approximately 37% of the administered dose of chlorpromazine is excreted in urine. Route of Elimination: Kidneys, ~ 37% excreted in urine Half Life: ~ 30 hours Biological Half-Life ~ 30 hours After 120 mg/sq m oral doses to human volunteers chlorpromazine displays a mean elimination half-life of approximately 18 hours (range, 6-119 hours). Disappearance of chlorpromazine from plasma includes rapid distribution phase (half-life about 2 hours) and slower early elimination phase (half-life about 30 hours), but markedly variable values have been reported; the half-life of elimination from human brain is not known. The pharmacokinetics of chlorpromazine in the newborn have not been reported. /Investigators/ studied the kinetics of removal of chlorpromazine from plasma in an infant whose mother was treated with high doses of chlorpromazine and lithium throughout the last trimester of pregnancy. The infant exhibited symptoms of severe neurologic depression that slowly abated over the first 9 days of life. The kinetics of plasma chlorpromazine removal were described with a two-compartment model, exhibiting a rapid half-life of 1.46 days and a slow half-life of 3.19 days. Both half-lives are considerably longer than the rapid and slow half-lives described in adults. Caution in exposing the fetus or newborn to chlorpromazine is warranted. Further information on the distribution and excretion of chlorpromazine by the newborn is needed. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION: Chlorpromazine is an antipsychotic medication. It is a synthetic dimethylamine derivative of phenothiazine. Chlorpromazine is a white to creamy-white (Base and hydrochloride). Powder or waxy solid (Base); crystallline powder (Hydrochloride). Chlorpromzaine is practically insoluble in water. Freely soluble in dilute mineral acids; practically insoluble in dilute alkali hydroxides. HUMAN EXPOSURE: Main risks and target organs: The principal pharmacological actions are psychotropic. It also exerts sedative and antiemetic activity. Chlorpromazine has actions at all levels of the central nervous system, primarily at subcortical levels, as well as on multiple organ systems. Chlorpromazine has strong antiadrenergic and weak peripheral anticholinergic activity; ganglionic blocking action is relatively slight. It also possesses slight antihistaminic and antiserotonin activity. Summary of clinical effects: Central nervous depression may progress from drowsiness to coma, ultimately with areflexia. In early or mild intoxications, some patients suffer from restlessness, confusion and excitement. Tremor or muscular twitching, spasm, rigidity, convulsions, muscular hypotonia, difficulty in swallowing may be present. Extrapyramidal signs of overdose include dystonia, torticollis, oculogyric crises and opisthotonos. Either hypothermia or hyperthermia may be encountered. Difficulty in breathing, cyanosis, respiratory and/or vasomotor collapse, respiratory depression and distress, sudden apnea and even cyanosis may occur. Hypotension, tachycardia, cardiac arrhythmias, conduction defects, ventricular fibrillation or cardiac arrest may occur. Contraindications: Do not use in comatose states or in the presence of large amounts of central nervous system depressants (alcohol, barbiturates, anesthetics, narcotics, etc.) because chlorpromazine prolongs and intensifies the action of such CNS depressants. Chlorpromazine should be administered cautiously to persons with cardiovascular or liver disease. There is evidence that patients with a history of hepatic encephalopathy due to cirrhosis have increased sensitivity to the CNS effect of chlorpromazine (e.g. impaired cerebration and abnormal slowing of the EEG). Because of this CNS depressant effect, it should be used with caution in patients with chronic respiratory disorders such as severe asthma, emphysema and acute respiratory infections, particularly in children. Because it can suppress the cough reflex, aspiration of vomitus is possible. Subcutaneous injection is contraindicated. Routes of entry: Oral: Chlorpromazine is available in tablet or syrup forms for oral ingestion. Parenteral: It is present in injectable forms for use through the intramuscular or intravenous routes. Other: Rectal route with suppositories. Absorption by route of exposure: The absorption of orally administered chlorpromazine is dependent on the dosage form, the elixir giving the highest plasma concentration of drug. Peak plasma levels are reached at 2 to 3 hours. There is a wide inter-subject variability (ten times or more) in the plasma concentrations achieved. Plasma concentrations may be decreased significantly by food in the stomach and by the concomitant administration of anticholinergic antiparkinsonism drugs. Owing to the first-pass effect, plasma concentrations following oral administrations are much lower than those following intramuscular administrations. Distribution by route of exposure: Chlorpromazine is widely distributed in the body and crosses the blood-brain barrier to achieve higher concentrations in the brain than in the plasma. Chlorpromazine and its metabolites also cross the placental barrier and are excreted in milk. Chlorpromazine is highly bound to plasma proteins, varying from 91.8% to 97% over the range of clinical blood concentrations (0.01 to 1 mcg/mL). Binding is easily reversed. Biological half-life by route of exposure: Although the plasma half-life of chlorpromazine itself has been reported to be only a few hours, elimination of the metabolites may be very prolonged. Blood studies show a range of 2 to 3 days and for the urinary studies up to about 18 days. Chlorpromazine brings about changes that can persist much longer than these times after discontinuation of the drug. The exact relationship of persisting therapeutic effects to administered chlorpromazine is uncertain. There is the possibility that minute amounts of chlorpromazine and/or metabolites persist at active sites in slowly reversible or relatively irreversible ways. It also seems that some chlorpromazine is stored in adipose tissue and slowly mobilized after stopping chlorpromazine administration. Metabolism: Paths of metabolism of chlorpromazine include hydroxylation, and conjugation with glucuronic acid, N-oxidation, oxidation of a sulfur atom, and dealkylation. In man, after chronic use, the highest concentration of unconjugated chlorpromazine metabolites is found in the lung and liver. The 7-hydroxy chlorpromazine that is found in body tissues appears to be an active metabolite. Since there is some evidence that chlorpromazine can cause hepatic microsomal enzyme induction, it may accelerate its own metabolism; this may account for progressively decreasing plasma concentrations of free drug during maintenance of a fixed dosage schedule. One hundred and sixty-eight possible metabolites of chlorpromazine have been postulated and many of them actually isolated from human urine. In man, urinary excretion of chlorpromazine plus its sulfoxides varies from 1 to 20% of the daily dose administered. The average ratio of free chlorpromazine to the sulfoxide in the urine is about 1:16. There is much evidence that the sulfoxide undergoes additional metabolism, probably to sulfones. The various phenothiazine congeners of chlorpromazine undergo similar metabolic degradation. Demethylation is another method of detoxication by the liver. Elimination by route of exposure: Chlorpromazine is excreted in both urine and feces. Mode of action: Chlorpromazine has a wide range of activity arising from its depressant actions on the central nervous system and its alpha-adrenergic blocking and weak antimuscarinic activities. Chlorpromazine possesses sedative properties but patients usually develop tolerance rapidly to the sedation. Its action on the autonomic system produces vasodilation, hypotension, and tachycardia. Salivary and gastric secretions are reduced. The sulfoxides of the phenothiazines have been intensively studied and found to be significantly less potent than the parent compound. Teratogenicity: If given in high doses over a long period during pregnancy, chlorpromazine may cause damage to the retina of the fetus. Interactions: Chlorpromazine may block the antihypertensive effects of guanethidine. Patients being treated with phenothiazines should be advised that their susceptibility to alcohol may be increased. Chlorpromazine has been shown to increase the miotic and sedative effects of morphine. Chlorpromazine may enhance the respiratory depression produced particularly by CNS depressants. Mutual inhibition of liver enzymes concerned with the metabolism of both chlorpromazine and the other drug (e.g. a tricyclic antidepressant) might result in increased plasma-concentrations of either drug. Chlorpromazine is reported to interfere with a number of laboratory tests, such as pregnancy tests, thyroid function tests, the Coombs' test where a false positive result can be achieved, and adrenal medullary tests. It is also reported to interfere with estimations for serum 5-hydroxyindole-acetic acid, blood urea, urinary ketones and steroids, urinary porphobilinogen, and vitamin B12. Main adverse effects: Therapeutic doses of chlorpromazine, may cause palpitation, nasal stuffiness, dry mouth, and slight constipation. The patient may complain of being cold, drowsy, or weak. Orthostatic hypotension, which may result in syncope. A mild elevation of temperature may be seen during the first few days, particularly if the drug is given parenterally. On the other hand, hypothermia can occur and may be due both to the action on the heat regulating center and to direct peripheral vasodilation. Sensitivity and adaptation to environmental temperature change are impaired so that fatal hyperthermia and heat stroke are possible complications. Chlorpromazine has produced hematological disorders, including agranulocytosis, eosinophilia, leucopenia, hemolytic anemia, aplastic anemia, thrombocytopenic purpura and pancytopenia. Hyperglycemia, hypoglycemia and glycosuria have also been reported. Chlorpromazine acts as an antagonist (blocking agent) on different postsysnaptic receptors -on dopaminergic-receptors (subtypes D1, D2, D3 and D4 - different antipsychotic properties on productive and unproductive symptoms), on serotonergic-receptors (5-HT1 and 5-HT2, with anxiolytic, antidepressive and antiaggressive properties as well as an attenuation of extrapypramidal side-effects, but also leading to weight gain, fall in blood pressure, sedation and ejaculation difficulties), on histaminergic-receptors (H1-receptors, sedation, antiemesis, vertigo, fall in blood pressure and weight gain), alpha1/alpha2-receptors (antisympathomimetic properties, lowering of blood pressure, reflex tachycardia, vertigo, sedation, hypersalivation and incontinence as well as sexual dysfunction, but may also attenuate pseudoparkinsonism - controversial) and finally on muscarinic (cholinergic) M1/M2-receptors (causing anticholinergic symptoms like dry mouth, blurred vision, obstipation, difficulty/inability to urinate, sinus tachycardia, ECG-changes and loss of memory, but the anticholinergic action may attenuate extrapyramidal side-effects). Additionally, Chlorpromazine is a weak presynaptic inhibitor of Dopamine reuptake, which may lead to (mild) antidepressive and antiparkinsonian effects. This action could also account for psychomotor agitation and amplification of psychosis (very rarely noted in clinical use). Interactions ... Dosage requirements for oral hypoglycemic agents may be increased in those receiving chlorpromazine ... . QT interval-prolonging medications, including cisapride, erythromycin, and quinidine /may produce/ additive QT interval prolongation increasing the risk of developing cardiac arrhythmias when /concurrently administered with phenothiazines/. /Phenothiazines/ Concurrent use /of other photosensitizing medications/ with phenothiazines may cause additive photosensitizing effects. In addition, concurrent use of systemic methoxsalen, trixsalen, or tetracyclines with phenothiazines may potentiate intraocular photochemical damage to the choroid, retina, or lens. /Phenothiazines/ Prior administration of phenothiazines may decrease the pressor effect and shorten the duration of action of phenylephrine. /Phenothiazines/ For more Interactions (Complete) data for Chlorpromazine (37 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat oral 225 mg/kg LD50 Rat oral 142 mg/kg LD50 Rat ip 58 mg/kg LD50 Rat sc 75 mg/kg For more Non-Human Toxicity Values (Complete) data for Chlorpromazine (10 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Antiemetics; Antipsychotic Agents, Phenothiazine; Dopamine Antagonists /Chlorpromazine is indicated/ for the treatment of schizophrenia. /Included in US product label/ /Chlorpromazine is indicated/ to control nausea and vomiting. /Included in US product label/ /Chlorpromazine is indicated/ for relief of restlessness and apprehension before surgery. /Included in US product label/ For more Therapeutic Uses (Complete) data for Chlorpromazine (16 total), please visit the HSDB record page. Drug Warnings Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Chlorpromazine Hydrochloride Injection, USP is not approved for the treatment of patients with dementia-related psychosis. ... Extrapyramidal reactions ... fairly common, usually 3 types ... Parkinsonian-like syndrome ... dystonia and dyskinesia, including torticollis, tics, and other involuntary muscle movements ... akathisia, shown by restlessness ... hyperreflexia, reported in newborn ... ./Phenothiazines/ The antiemetic action of chlorpromazine may mask the signs and symptoms of overdosage of other drugs and may obscure the diagnosis and treatment of other conditions such as intestinal obstruction, brain tumor and Reye's syndrome. When chlorpromazine is used with cancer chemotherapeutic drugs, vomiting as a sign of the toxicity of these agents may be obscured by the antiemetic effects of chlorpromazine. Do not use /chlorpromazine/ in patients with known hypersensitivity to phenothiazines. Do not use in comatose states or in the presence of large amounts of central nervous system depressants (alcohol, barbiturates, narcotics, etc.). For more Drug Warnings (Complete) data for Chlorpromazine (55 total), please visit the HSDB record page. Pharmacodynamics Chlorpromazine is a psychotropic agent indicated for the treatment of schizophrenia. It also exerts sedative and antiemetic activity. Chlorpromazine has actions at all levels of the central nervous system-primarily at subcortical levels-as well as on multiple organ systems. Chlorpromazine has strong antiadrenergic and weaker peripheral anticholinergic activity; ganglionic blocking action is relatively slight. It also possesses slight antihistaminic and antiserotonin activity. |
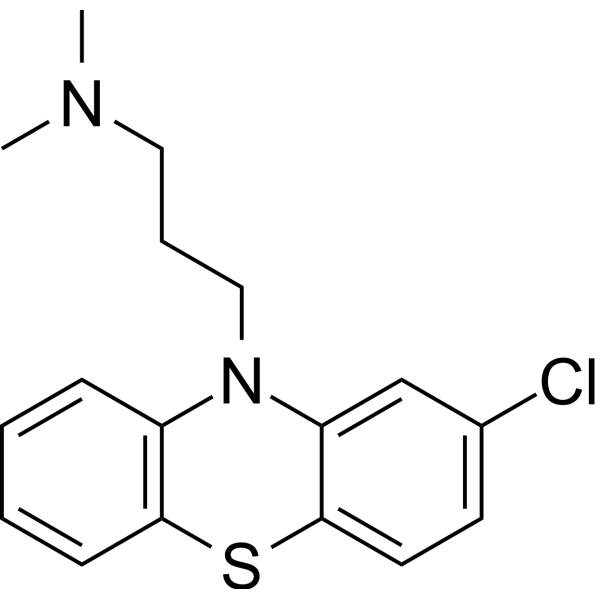
| 分子式 |
C17H19CLN2S
|
|---|---|
| 分子量 |
318.864161729813
|
| 精确质量 |
318.095
|
| 元素分析 |
C, 64.04; H, 6.01; Cl, 11.12; N, 8.79; S, 10.05
|
| CAS号 |
50-53-3
|
| 相关CAS号 |
Chlorpromazine hydrochloride;69-09-0;Chlorpromazine-d6 hydrochloride;1228182-46-4;Chlorpromazine-d6 oxalate;1276197-23-9
|
| PubChem CID |
2726
|
| 外观&性状 |
White to off-white solid
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
450.1±45.0 °C at 760 mmHg
|
| 熔点 |
192 - 196ºC (decomposes)
|
| 闪点 |
226.0±28.7 °C
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
| 折射率 |
1.623
|
| LogP |
5.2
|
| tPSA |
31.78
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
21
|
| 分子复杂度/Complexity |
339
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CN(C)CCCN1C2=CC=CC=C2SC3=C1C=C(C=C3)Cl
|
| InChi Key |
ZPEIMTDSQAKGNT-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3
|
| 化学名 |
10H-Phenothiazine-10-propanamine, 2-chloro-N,N-dimethyl-
|
| 别名 |
BC-135; BC135; BC 135
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: (1). 本产品在运输和储存过程中需避光。 (2). 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 100 mg/mL (313.62 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.84 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (7.84 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (7.84 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1362 mL | 15.6809 mL | 31.3617 mL | |
| 5 mM | 0.6272 mL | 3.1362 mL | 6.2723 mL | |
| 10 mM | 0.3136 mL | 1.5681 mL | 3.1362 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。