| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| Other Sizes |

| 靶点 |
D2 dopamine receptors (Ki = 363 nM ); 5-HT2A (Ki = 8.3 nM)
Dopamine D2 receptor (D2R) (human D2R, Ki=2.3 nM; rat D2R, Ki=3.1 nM) [1,2] Histamine H1 receptor (H1R) (Ki=8.5 nM) [1] Muscarinic cholinergic receptors (M1-M3) (M1: Ki=420 nM; M2: Ki=380 nM; M3: Ki=450 nM) [1] Calmodulin (CaM) (IC50=15 μM) [2] |
|---|---|
| 体外研究 (In Vitro) |
氯丙嗪通过降低 GABAAR 的结合率 (kon) 和增加解除结合率 (koff) 来影响微型 IPSC (mIPSC)。氯丙嗪以电压依赖性方式调节激活的 TRPA1 电流,导致正电位阻断,负电位开放概率增加。
多巴胺(10 μM)刺激的大鼠纹状体神经元经盐酸氯丙嗪(Chlorpromazine HCl)(0.1 μM-10 μM)处理后,药物剂量依赖性抑制D2R介导的cAMP积累,IC50=2.8 μM,5 μM时神经元放电频率降低65%[1] - 刀豆蛋白A(Con A,5 μg/mL)激活的人T淋巴细胞经盐酸氯丙嗪(Chlorpromazine HCl)(1 μM-50 μM)处理后,抑制T细胞增殖(IC50=12 μM),20 μM时ELISA检测显示IL-2/IFN-γ分泌分别减少60%/55%[3] - LPS(1 μg/mL)诱导的小鼠腹腔巨噬细胞经盐酸氯丙嗪(Chlorpromazine HCl)(5 μM-30 μM)处理后,20 μM时抑制TNF-α/IL-6释放70%/68%,免疫荧光检测显示NF-κB核转位受抑58%[4] - 钙调蛋白依赖性磷酸二酯酶(CaM-PDE)活性实验显示,盐酸氯丙嗪(Chlorpromazine HCl)(10 μM-100 μM)抑制酶活性,IC50=15 μM,机制为直接结合CaM[2] - 原代大鼠皮质神经元经盐酸氯丙嗪(Chlorpromazine HCl)(1 μM-20 μM)处理后,10 μM时减少42%的电压门控钙电流,无显著神经元毒性[5] |
| 体内研究 (In Vivo) |
在体内模型中,氯丙嗪独立下调各种 T 细胞来源的淋巴因子(IL-2、IFN-gamma、IL-4、TNF 和 GM-CSF)的产生并上调 IL-10 的分泌。急性超抗原驱动的免疫激活。氯丙嗪介导的SEB驱动的氯丙嗪分泌放大伴随着IL-10 mRNA积累的增强。氯丙嗪可保护正常小鼠或肾上腺切除小鼠以及豚鼠免受 LPS 的致死作用,并抑制 TNF 血清水平。在 LPS 前 30 分钟 (min)、同时或后 10 分钟内施用氯丙嗪可防止 LPS 致死,而在 LPS 后 30 分钟施用则无效,与对 TNF 产生的抑制作用相似。氯丙嗪显着抑制对放线菌素 D 的 LPS 毒性敏感的小鼠的 LPS 致死率和肝毒性,而在这些条件下,DEX 不活跃。氯丙嗪可保护大鼠脑组织免受缺氧引起的不可逆突触传递损失。氯丙嗪还能显着延缓大鼠缺氧引起的扩散性抑郁症(SD)的发生。
小鼠苯丙胺诱导多动模型:腹腔注射盐酸氯丙嗪(Chlorpromazine HCl)(0.5 mg/kg、1 mg/kg、2 mg/kg),30分钟后皮下注射苯丙胺(5 mg/kg)。2 mg/kg剂量时减少75%的自发活动量,使纹状体多巴胺周转率恢复正常[1] - 大鼠佐剂诱导关节炎模型:口服灌胃盐酸氯丙嗪(Chlorpromazine HCl)(10 mg/kg、20 mg/kg),每日一次,连续14天。20 mg/kg剂量时减轻62%的足肿胀,减少滑膜组织中58%的TNF-α和60%的IL-6[4] - 兔条件性回避反应(CAR)模型:静脉注射盐酸氯丙嗪(Chlorpromazine HCl)(1 mg/kg、3 mg/kg),1 mg/kg剂量抑制CAR 45%,3 mg/kg剂量抑制80%,该反应是抗精神病活性的标志[5] - 小鼠接触性超敏反应模型:二硝基氟苯(DNFB)致敏后24小时,在耳皮肤局部涂抹盐酸氯丙嗪(Chlorpromazine HCl)(0.5% w/v),减轻55%的耳肿胀,抑制CD4+ T细胞浸润[3] |
| 酶活实验 |
最近的研究强调,突触后GABAA受体(GABAAR)激活的非平衡条件是形成IPSCs时间进程的关键因素(Puia等人,1994;Jones和Westbrook,1995年)。这种非平衡是由极快的激动剂时间过程引起的,可能会影响药理学药物和突触后GABAARs之间的相互作用。在本研究中,我们发现氯丙嗪(CPZ)是一种广泛使用的抗精神病药物,已知会干扰几种配体和电压门控通道,可降低小型IPSCs(mIPSCs)的振幅并加速其衰变。当通过对切除的贴片的超快GABA应用的反应来模拟mIPSCs时,获得了CPZ对mIPSCs的影响的良好定性再现。我们的实验数据和模型模拟表明,CPZ通过降低GABAARs的结合率(kon)和增加去结合率(koff)来影响mIPSCs。由于CPZ对kon的减少,结合反应变得限速,并且在mIPSC期间GABAARs的激动剂暴露时间太短,无法像在对照条件下那样激活受体。解束缚率的增加被认为是mIPSC衰减阶段加速的潜在机制。CPZ对GABAAR结合率的影响,导致GABA诱发电流的缓慢启动,为估计GABA的突触清除速度提供了一种工具。此外,记录的反应的起始动力学允许估计突触GABA的峰值浓度[1]。
D2R结合实验:从表达人/大鼠D2R的HEK293细胞或大鼠纹状体组织制备膜组分,将膜样品与[3H]-螺哌隆(0.5 nM)及不同浓度的盐酸氯丙嗪(Chlorpromazine HCl)(0.01 nM-100 nM)在25°C孵育60分钟。通过真空过滤玻璃纤维滤膜分离结合态和游离态配体,用液体闪烁计数器测量放射性,采用Cheng-Prusoff方程计算Ki值[1] - CaM结合实验:将纯化的CaM(1 μM)与盐酸氯丙嗪(Chlorpromazine HCl)(1 μM-100 μM)在缓冲液中37°C孵育30分钟,加入CaM-PDE底物,分光光度法检测酶活性,计算CaM抑制的IC50[2] - H1R结合实验:从表达人H1R的CHO细胞制备膜组分,将膜样品与[3H]-吡拉明(0.5 nM)及盐酸氯丙嗪(Chlorpromazine HCl)(0.1 nM-1 μM)在25°C孵育60分钟。真空过滤分离结合态/游离态配体,测量放射性并计算Ki值[1] |
| 细胞实验 |
细胞系:U-87MG 胶质瘤细胞
浓度:0、10、20、40 μM 孵育时间:0、24、48 小时 结果:在剂量和时间范围内显示出抗增殖活性依赖方式。 T细胞增殖实验:密度梯度离心法分离人外周血T淋巴细胞,接种于96孔板,用Con A(5 μg/mL)激活,加入盐酸氯丙嗪(Chlorpromazine HCl)(1 μM-50 μM)处理72小时。MTT法评估增殖;收集上清液ELISA法定量IL-2/IFN-γ[3] - 巨噬细胞细胞因子分泌实验:腹腔灌洗法分离小鼠腹腔巨噬细胞,接种于24孔板,用盐酸氯丙嗪(Chlorpromazine HCl)(5 μM-30 μM)预处理1小时,再用LPS(1 μg/mL)刺激24小时。收集上清液ELISA法定量TNF-α/IL-6;免疫荧光检测NF-κB核转位[4] - 神经元钙电流实验:原代大鼠皮质神经元接种于96孔板培养7天,采用膜片钳技术记录药物处理前后(盐酸氯丙嗪(Chlorpromazine HCl)1 μM-20 μM)的电压门控钙电流,分析电流振幅和峰值电压[5] |
| 动物实验 |
5- to 6-week-old athymic nude mice bearing intracranial U-87MG xenograft tumors[2]
20 mg/kg Injected intraperitoneally; single daily for 7 days Amphetamine-induced hyperactivity model: Male ICR mice (20-25 g) were acclimated to activity chambers for 30 minutes. Chlorpromazine HCl was dissolved in physiological saline and administered via intraperitoneal injection (0.5 mg/kg, 1 mg/kg, 2 mg/kg) 30 minutes before subcutaneous injection of amphetamine (5 mg/kg). Record locomotor activity for 120 minutes post-amphetamine [1] - Adjuvant-induced arthritis model: Male Wistar rats (200-250 g) were injected with complete Freund's adjuvant (0.1 mL) into the hind paw to induce arthritis. From day 7 post-induction, Chlorpromazine HCl was dissolved in 0.5% carboxymethylcellulose sodium and administered via oral gavage (10 mg/kg, 20 mg/kg) daily for 14 days. Measure paw volume every 3 days; euthanize rats to collect synovial tissue for cytokine detection [4] - Conditioned avoidance response model: Male New Zealand white rabbits (2.0-2.5 kg) were trained to avoid electric shock via conditioned response. Chlorpromazine HCl was dissolved in physiological saline and administered via intravenous injection (1 mg/kg, 3 mg/kg) 30 minutes before the test. Record avoidance success rate over 60 minutes [5] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Readily absorbed from the GI tract. Bioavailability varies due to first-pass metabolism by the liver. Kidneys, ~ 37% excreted in urine 20 L/kg Chlorpromazine hydrochloride is rapidly absorbed from the GI tract and from parenteral sites of injection; however, following oral administration, the drug undergoes considerable metabolism during absorption (in the GI mucosa) and first pass through the liver. Although not clearly established in humans, chlorpromazine and its metabolites undergo enterohepatic circulation in animals. Considerable interindividual variations in peak plasma concentrations have been reported with the same oral dose of chlorpromazine. The variability is thought to result from wide interindividual variation in bioavailability, apparently because of genetic differences in the rate of first-pass metabolism. As a result of first-pass metabolism, less chlorpromazine reaches systemic circulation as unchanged drug, and peak plasma chlorpromazine concentrations are much lower following oral administration than following im administration. Following oral administration of chlorpromazine hydrochloride in a tablet formulation, the onset of pharmacologic action occurs within 30-60 minutes; the duration of action is 4-6 hours. The onset of pharmacologic action following oral administration of chlorpromazine hydrochloride in an extended-release formulation is approximately 30-60 minutes; the duration of action is 10-12 hours. The onset of pharmacologic action following rectal administration of chlorpromazine is generally slower than that following oral administration of chlorpromazine hydrochloride; rectally administered chlorpromazine has a duration of action of 3-4 hours. Phenothiazines and their metabolites are distributed into most body tissues and fluids, with high concentrations being distributed into the brain, lungs, liver, kidneys, and spleen. /Phenothiazine General Statement/ For more Absorption, Distribution and Excretion (Complete) data for Chlorpromazine (17 total), please visit the HSDB record page. Metabolism / Metabolites Extensively metabolized in the liver and kidneys. It is extensively metabolized by cytochrome P450 isozymes CYP2D6 (major pathway), CYP1A2 and CYP3A4. Approximately 10 to 12 major metabolite have been identified. Hydroxylation at positions 3 and 7 of the phenothiazine nucleus and the N-dimethylaminopropyl side chain undergoes demethylation and is also metabolized to an N-oxide. In urine, 20% of chlopromazine and its metabolites are excreted unconjugated in the urine as unchanged drug, demonomethylchlorpromazine, dedimethylchlorpromazine, their sulfoxide metabolites, and chlorpromazine-N-oxide. The remaining 80% consists of conjugated metabolites, principally O-glucuronides and small amounts of ethereal sulfates of the mono- and dihydroxy-derivatives of chlorpromazine and their sulfoxide metabolites. The major metabolites are the monoglucuronide of N-dedimethylchlorpromazine and 7-hydroxychlorpromazine. Approximately 37% of the administered dose of chlorpromazine is excreted in urine. Although the exact metabolic fate of chlorpromazine is not clearly established, the drug is extensively metabolized, principally in the liver and kidneys. About 10-12 metabolites which occur in humans in appreciable quantities have been identified. In addition to hydroxylation at positions 3 and 7 of the phenothiazine nucleus, the N-dimethylaminopropyl side chain of chlorpromazine undergoes demethylation and is also metabolized to an N-oxide. Two principal groups of metabolites have been found in urine. The unconjugated fraction, which represents approximately 20% of chlorpromazine and its metabolites excreted in urine, consists of unchanged drug, demonomethylchlorpromazine, dedimethylchlorpromazine, their sulfoxide metabolites, and chlorpromazine-N-oxide. The conjugated fraction, which represents approximately 80% of chlorpromazine and its metabolites excreted in urine, consists principally of O-glucuronides, with small amounts of ethereal sulfates of the mono- and dihydroxy-derivatives of chlorpromazine and their sulfoxide metabolites. The major metabolites found in urine are the monoglucuronide of N-dedimethylchlorpromazine and 7-hydroxychlorpromazine. Most metabolites of phenothiazines are pharmacologically inactive; however, certain metabolites (eg, 7-hydroxychlorpromazine, mesoridazine) show moderate pharmacologic activity and may contribute to the action of the drugs. There is limited evidence to indicate that some phenothiazines (eg, chlorpromazine) may induce their own metabolism. /Phenothiazine General Statement/ Yields 2-chlorophenothiazine in man; promazine probably in dog and in man ... . Yields demethylchlorpromazine in man, rat, rabbit, mouse, dog, sheep, guinea pig ... . Yields chlorpromazine sulfoxide in man, rat, rabbit, dog ... . Yields chlorpromazine-n-oxide in man, rat, rabbit, dog, pig, sheep, guinea pig, mouse ... . Yields 3-hydroxychlorpromazine in man, rat, dog ... . Yields 7-hydroxychlorpromazine in man, rat, sheep, dog, rabbit, guinea pig ... . /From table/ As least 10 or 12 metabolites of chlorpromazine occur in human beings in appreciable quantities. Quantitatively, the most important of these are nor2-chlorpromazine (doubly methylated), chlorphenothiazine (removal of the entire side chain), methoxy and hydroxy products, and glucuronide conjugates of the hydroxylated compounds. In urine, 7-hydroxylated and dealkylated (nor2) metabolites and their conjugates predominate. Chlorpromazine has known human metabolites that include 7-Hydroxychlorpromazine, Chlorpromazine S-oxide, Chlorpromazine, N-desmethyl, and Chlorpromazine N-glucuronide. Extensively metabolized in the liver and kidneys. It is extensively metabolized by cytochrome P450 isozymes CYP2D6 (major pathway), CYP1A2 and CYP3A4. Approximately 10 to 12 major metabolite have been identified. Hydroxylation at positions 3 and 7 of the phenothiazine nucleus and the N-dimethylaminopropyl side chain undergoes demethylation and is also metabolized to an N-oxide. In urine, 20% of chlopromazine and its metabolites are excreted unconjugated in the urine as unchanged drug, demonomethylchlorpromazine, dedimethylchlorpromazine, their sulfoxide metabolites, and chlorpromazine-N-oxide. The remaining 80% consists of conjugated metabolites, principally O-glucuronides and small amounts of ethereal sulfates of the mono- and dihydroxy-derivatives of chlorpromazine and their sulfoxide metabolites. The major metabolites are the monoglucuronide of N-dedimethylchlorpromazine and 7-hydroxychlorpromazine. Approximately 37% of the administered dose of chlorpromazine is excreted in urine. Route of Elimination: Kidneys, ~ 37% excreted in urine Half Life: ~ 30 hours Biological Half-Life ~ 30 hours After 120 mg/sq m oral doses to human volunteers chlorpromazine displays a mean elimination half-life of approximately 18 hours (range, 6-119 hours). Disappearance of chlorpromazine from plasma includes rapid distribution phase (half-life about 2 hours) and slower early elimination phase (half-life about 30 hours), but markedly variable values have been reported; the half-life of elimination from human brain is not known. The pharmacokinetics of chlorpromazine in the newborn have not been reported. /Investigators/ studied the kinetics of removal of chlorpromazine from plasma in an infant whose mother was treated with high doses of chlorpromazine and lithium throughout the last trimester of pregnancy. The infant exhibited symptoms of severe neurologic depression that slowly abated over the first 9 days of life. The kinetics of plasma chlorpromazine removal were described with a two-compartment model, exhibiting a rapid half-life of 1.46 days and a slow half-life of 3.19 days. Both half-lives are considerably longer than the rapid and slow half-lives described in adults. Caution in exposing the fetus or newborn to chlorpromazine is warranted. Further information on the distribution and excretion of chlorpromazine by the newborn is needed. Absorption: Oral bioavailability is 30-40% in humans; peak plasma concentration (Cmax) is reached at 2-4 hours post-oral administration (100 mg dose: Cmax=450 ng/mL) [1] - Distribution: Volume of distribution (Vd) is 20-30 L/kg in humans; high blood-brain barrier penetration (brain/plasma concentration ratio=10-15) [1] - Metabolism: Extensively metabolized in the liver via cytochrome P450 (CYP) 2D6, 3A4, and 1A2 to active (7-hydroxychlorpromazine) and inactive metabolites [1] - Excretion: 70% of metabolites are excreted in urine, 25% in feces. Elimination half-life (t1/2) is 24-30 hours in humans [1] - Plasma protein binding: Chlorpromazine HCl has a plasma protein binding rate of 92-96% in human plasma [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION: Chlorpromazine is an antipsychotic medication. It is a synthetic dimethylamine derivative of phenothiazine. Chlorpromazine is a white to creamy-white (Base and hydrochloride). Powder or waxy solid (Base); crystallline powder (Hydrochloride). Chlorpromzaine is practically insoluble in water. Freely soluble in dilute mineral acids; practically insoluble in dilute alkali hydroxides. HUMAN EXPOSURE: Main risks and target organs: The principal pharmacological actions are psychotropic. It also exerts sedative and antiemetic activity. Chlorpromazine has actions at all levels of the central nervous system, primarily at subcortical levels, as well as on multiple organ systems. Chlorpromazine has strong antiadrenergic and weak peripheral anticholinergic activity; ganglionic blocking action is relatively slight. It also possesses slight antihistaminic and antiserotonin activity. Summary of clinical effects: Central nervous depression may progress from drowsiness to coma, ultimately with areflexia. In early or mild intoxications, some patients suffer from restlessness, confusion and excitement. Tremor or muscular twitching, spasm, rigidity, convulsions, muscular hypotonia, difficulty in swallowing may be present. Extrapyramidal signs of overdose include dystonia, torticollis, oculogyric crises and opisthotonos. Either hypothermia or hyperthermia may be encountered. Difficulty in breathing, cyanosis, respiratory and/or vasomotor collapse, respiratory depression and distress, sudden apnea and even cyanosis may occur. Hypotension, tachycardia, cardiac arrhythmias, conduction defects, ventricular fibrillation or cardiac arrest may occur. Contraindications: Do not use in comatose states or in the presence of large amounts of central nervous system depressants (alcohol, barbiturates, anesthetics, narcotics, etc.) because chlorpromazine prolongs and intensifies the action of such CNS depressants. Chlorpromazine should be administered cautiously to persons with cardiovascular or liver disease. There is evidence that patients with a history of hepatic encephalopathy due to cirrhosis have increased sensitivity to the CNS effect of chlorpromazine (e.g. impaired cerebration and abnormal slowing of the EEG). Because of this CNS depressant effect, it should be used with caution in patients with chronic respiratory disorders such as severe asthma, emphysema and acute respiratory infections, particularly in children. Because it can suppress the cough reflex, aspiration of vomitus is possible. Subcutaneous injection is contraindicated. Routes of entry: Oral: Chlorpromazine is available in tablet or syrup forms for oral ingestion. Parenteral: It is present in injectable forms for use through the intramuscular or intravenous routes. Other: Rectal route with suppositories. Absorption by route of exposure: The absorption of orally administered chlorpromazine is dependent on the dosage form, the elixir giving the highest plasma concentration of drug. Peak plasma levels are reached at 2 to 3 hours. There is a wide inter-subject variability (ten times or more) in the plasma concentrations achieved. Plasma concentrations may be decreased significantly by food in the stomach and by the concomitant administration of anticholinergic antiparkinsonism drugs. Owing to the first-pass effect, plasma concentrations following oral administrations are much lower than those following intramuscular administrations. Distribution by route of exposure: Chlorpromazine is widely distributed in the body and crosses the blood-brain barrier to achieve higher concentrations in the brain than in the plasma. Chlorpromazine and its metabolites also cross the placental barrier and are excreted in milk. Chlorpromazine is highly bound to plasma proteins, varying from 91.8% to 97% over the range of clinical blood concentrations (0.01 to 1 mcg/mL). Binding is easily reversed. Biological half-life by route of exposure: Although the plasma half-life of chlorpromazine itself has been reported to be only a few hours, elimination of the metabolites may be very prolonged. Blood studies show a range of 2 to 3 days and for the urinary studies up to about 18 days. Chlorpromazine brings about changes that can persist much longer than these times after discontinuation of the drug. The exact relationship of persisting therapeutic effects to administered chlorpromazine is uncertain. There is the possibility that minute amounts of chlorpromazine and/or metabolites persist at active sites in slowly reversible or relatively irreversible ways. It also seems that some chlorpromazine is stored in adipose tissue and slowly mobilized after stopping chlorpromazine administration. Metabolism: Paths of metabolism of chlorpromazine include hydroxylation, and conjugation with glucuronic acid, N-oxidation, oxidation of a sulfur atom, and dealkylation. In man, after chronic use, the highest concentration of unconjugated chlorpromazine metabolites is found in the lung and liver. The 7-hydroxy chlorpromazine that is found in body tissues appears to be an active metabolite. Since there is some evidence that chlorpromazine can cause hepatic microsomal enzyme induction, it may accelerate its own metabolism; this may account for progressively decreasing plasma concentrations of free drug during maintenance of a fixed dosage schedule. One hundred and sixty-eight possible metabolites of chlorpromazine have been postulated and many of them actually isolated from human urine. In man, urinary excretion of chlorpromazine plus its sulfoxides varies from 1 to 20% of the daily dose administered. The average ratio of free chlorpromazine to the sulfoxide in the urine is about 1:16. There is much evidence that the sulfoxide undergoes additional metabolism, probably to sulfones. The various phenothiazine congeners of chlorpromazine undergo similar metabolic degradation. Demethylation is another method of detoxication by the liver. Elimination by route of exposure: Chlorpromazine is excreted in both urine and feces. Mode of action: Chlorpromazine has a wide range of activity arising from its depressant actions on the central nervous system and its alpha-adrenergic blocking and weak antimuscarinic activities. Chlorpromazine possesses sedative properties but patients usually develop tolerance rapidly to the sedation. Its action on the autonomic system produces vasodilation, hypotension, and tachycardia. Salivary and gastric secretions are reduced. The sulfoxides of the phenothiazines have been intensively studied and found to be significantly less potent than the parent compound. Teratogenicity: If given in high doses over a long period during pregnancy, chlorpromazine may cause damage to the retina of the fetus. Interactions: Chlorpromazine may block the antihypertensive effects of guanethidine. Patients being treated with phenothiazines should be advised that their susceptibility to alcohol may be increased. Chlorpromazine has been shown to increase the miotic and sedative effects of morphine. Chlorpromazine may enhance the respiratory depression produced particularly by CNS depressants. Mutual inhibition of liver enzymes concerned with the metabolism of both chlorpromazine and the other drug (e.g. a tricyclic antidepressant) might result in increased plasma-concentrations of either drug. Chlorpromazine is reported to interfere with a number of laboratory tests, such as pregnancy tests, thyroid function tests, the Coombs' test where a false positive result can be achieved, and adrenal medullary tests. It is also reported to interfere with estimations for serum 5-hydroxyindole-acetic acid, blood urea, urinary ketones and steroids, urinary porphobilinogen, and vitamin B12. Main adverse effects: Therapeutic doses of chlorpromazine, may cause palpitation, nasal stuffiness, dry mouth, and slight constipation. The patient may complain of being cold, drowsy, or weak. Orthostatic hypotension, which may result in syncope. A mild elevation of temperature may be seen during the first few days, particularly if the drug is given parenterally. On the other hand, hypothermia can occur and may be due both to the action on the heat regulating center and to direct peripheral vasodilation. Sensitivity and adaptation to environmental temperature change are impaired so that fatal hyperthermia and heat stroke are possible complications. Chlorpromazine has produced hematological disorders, including agranulocytosis, eosinophilia, leucopenia, hemolytic anemia, aplastic anemia, thrombocytopenic purpura and pancytopenia. Hyperglycemia, hypoglycemia and glycosuria have also been reported. Chlorpromazine acts as an antagonist (blocking agent) on different postsysnaptic receptors -on dopaminergic-receptors (subtypes D1, D2, D3 and D4 - different antipsychotic properties on productive and unproductive symptoms), on serotonergic-receptors (5-HT1 and 5-HT2, with anxiolytic, antidepressive and antiaggressive properties as well as an attenuation of extrapypramidal side-effects, but also leading to weight gain, fall in blood pressure, sedation and ejaculation difficulties), on histaminergic-receptors (H1-receptors, sedation, antiemesis, vertigo, fall in blood pressure and weight gain), alpha1/alpha2-receptors (antisympathomimetic properties, lowering of blood pressure, reflex tachycardia, vertigo, sedation, hypersalivation and incontinence as well as sexual dysfunction, but may also attenuate pseudoparkinsonism - controversial) and finally on muscarinic (cholinergic) M1/M2-receptors (causing anticholinergic symptoms like dry mouth, blurred vision, obstipation, difficulty/inability to urinate, sinus tachycardia, ECG-changes and loss of memory, but the anticholinergic action may attenuate extrapyramidal side-effects). Additionally, Chlorpromazine is a weak presynaptic inhibitor of Dopamine reuptake, which may lead to (mild) antidepressive and antiparkinsonian effects. This action could also account for psychomotor agitation and amplification of psychosis (very rarely noted in clinical use). Interactions ... Dosage requirements for oral hypoglycemic agents may be increased in those receiving chlorpromazine ... . QT interval-prolonging medications, including cisapride, erythromycin, and quinidine /may produce/ additive QT interval prolongation increasing the risk of developing cardiac arrhythmias when /concurrently administered with phenothiazines/. /Phenothiazines/ Concurrent use /of other photosensitizing medications/ with phenothiazines may cause additive photosensitizing effects. In addition, concurrent use of systemic methoxsalen, trixsalen, or tetracyclines with phenothiazines may potentiate intraocular photochemical damage to the choroid, retina, or lens. /Phenothiazines/ Prior administration of phenothiazines may decrease the pressor effect and shorten the duration of action of phenylephrine. /Phenothiazines/ For more Interactions (Complete) data for Chlorpromazine (37 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat oral 225 mg/kg LD50 Rat oral 142 mg/kg LD50 Rat ip 58 mg/kg LD50 Rat sc 75 mg/kg For more Non-Human Toxicity Values (Complete) data for Chlorpromazine (10 total), please visit the HSDB record page. Acute toxicity: LD50 is 214 mg/kg (oral) in rats, 146 mg/kg (oral) in mice; LD50 (intraperitoneal) is 57 mg/kg in rats [5] - Chronic toxicity: Rats administered Chlorpromazine HCl (50 mg/kg/day, oral) for 6 months showed extrapyramidal symptoms, mild liver enzyme elevation (2.0-fold), and retinal pigment deposition [1] - Clinical side effects: Extrapyramidal symptoms (dystonia, parkinsonism, akathisia) in 30-40% of patients; anticholinergic side effects (dry mouth, blurred vision, constipation) in 25-30%; sedation (40-50%) and orthostatic hypotension (15-20%) [1,5] - Drug-drug interaction: Inhibits CYP2D6 and 3A4, increasing plasma concentration of substrates (e.g., fluoxetine, warfarin) by 35-50%; potentiates sedative effects of alcohol, benzodiazepines, and opioids [1] |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Antiemetics; Antipsychotic Agents, Phenothiazine; Dopamine Antagonists /Chlorpromazine is indicated/ for the treatment of schizophrenia. /Included in US product label/ /Chlorpromazine is indicated/ to control nausea and vomiting. /Included in US product label/ /Chlorpromazine is indicated/ for relief of restlessness and apprehension before surgery. /Included in US product label/ For more Therapeutic Uses (Complete) data for Chlorpromazine (16 total), please visit the HSDB record page. Drug Warnings Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Chlorpromazine Hydrochloride Injection, USP is not approved for the treatment of patients with dementia-related psychosis. ... Extrapyramidal reactions ... fairly common, usually 3 types ... Parkinsonian-like syndrome ... dystonia and dyskinesia, including torticollis, tics, and other involuntary muscle movements ... akathisia, shown by restlessness ... hyperreflexia, reported in newborn ... ./Phenothiazines/ The antiemetic action of chlorpromazine may mask the signs and symptoms of overdosage of other drugs and may obscure the diagnosis and treatment of other conditions such as intestinal obstruction, brain tumor and Reye's syndrome. When chlorpromazine is used with cancer chemotherapeutic drugs, vomiting as a sign of the toxicity of these agents may be obscured by the antiemetic effects of chlorpromazine. Do not use /chlorpromazine/ in patients with known hypersensitivity to phenothiazines. Do not use in comatose states or in the presence of large amounts of central nervous system depressants (alcohol, barbiturates, narcotics, etc.). For more Drug Warnings (Complete) data for Chlorpromazine (55 total), please visit the HSDB record page. Pharmacodynamics Chlorpromazine is a psychotropic agent indicated for the treatment of schizophrenia. It also exerts sedative and antiemetic activity. Chlorpromazine has actions at all levels of the central nervous system-primarily at subcortical levels-as well as on multiple organ systems. Chlorpromazine has strong antiadrenergic and weaker peripheral anticholinergic activity; ganglionic blocking action is relatively slight. It also possesses slight antihistaminic and antiserotonin activity. Chlorpromazine HCl is a first-generation typical antipsychotic drug with anti-inflammatory, immunosuppressive, and calmodulin-inhibitory activities [1,3,4,5] Its core mechanisms include competitive antagonism of central dopamine D2 receptors (primary antipsychotic effect), H1R and muscarinic receptor blockade, inhibition of calmodulin-dependent enzymes, and suppression of T cell/macrophage-mediated immune responses [1,2,3,4] Indications include schizophrenia (positive symptoms: hallucinations, delusions), bipolar disorder (manic episodes), and severe nausea/vomiting. It is also used off-label for intractable hiccups and severe behavioral disorders [1,5] High blood-brain barrier penetration enables central antipsychotic effects but contributes to extrapyramidal and sedative side effects [1] Long elimination half-life (24-30 hours) supports once-daily or twice-daily oral dosing for adults (100-400 mg daily, divided doses) [1] It exhibits immunosuppressive effects via inhibiting T cell proliferation and pro-inflammatory cytokine secretion, suggesting potential in autoimmune disease management (clinical validation needed) [3,4] Caution is required in patients with liver disease, cardiovascular disorders, and glaucoma; regular monitoring of liver function and hematological parameters is recommended during long-term use [1,5] |
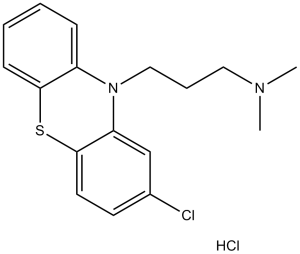
| 分子式 |
C17H20CL2N2S
|
|
|---|---|---|
| 分子量 |
355.33
|
|
| 精确质量 |
354.072
|
|
| 元素分析 |
C, 57.46; H, 5.67; Cl, 19.96; N, 7.88; S, 9.02
|
|
| CAS号 |
69-09-0
|
|
| 相关CAS号 |
Chlorpromazine; 50-53-3; Chlorpromazine-d6 hydrochloride; 1228182-46-4
|
|
| PubChem CID |
2726
|
|
| 外观&性状 |
White to off-white crystalline powder
|
|
| 密度 |
1.077 g/cm3 (15 C)
|
|
| 沸点 |
450.1ºC at 760 mmHg
|
|
| 熔点 |
192-196°C
|
|
| 折射率 |
1.4436 (20ºC)
|
|
| LogP |
5.761
|
|
| tPSA |
31.78
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
4
|
|
| 重原子数目 |
21
|
|
| 分子复杂度/Complexity |
339
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
ClC1C([H])=C([H])C2=C(C=1[H])N(C1=C([H])C([H])=C([H])C([H])=C1S2)C([H])([H])C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])[H]
|
|
| InChi Key |
FBSMERQALIEGJT-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C17H19ClN2S.ClH/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20;/h3-4,6-9,12H,5,10-11H2,1-2H3;1H
|
|
| 化学名 |
3-(2-chlorophenothiazin-10-yl)-N,N-dimethylpropan-1-amine;hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: (1). 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 (2). 该产品在溶液状态不稳定,请现配现用。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.04 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.85 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (5.85 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8143 mL | 14.0714 mL | 28.1429 mL | |
| 5 mM | 0.5629 mL | 2.8143 mL | 5.6286 mL | |
| 10 mM | 0.2814 mL | 1.4071 mL | 2.8143 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05190315 | Active Recruiting |
Radiation: Radiation Therapy Drug: Chlorpromazine Drug: Temozolomide |
Glioblastoma Multiforme | Mohammed Milhem | January 28, 2022 | Phase 1 |
| NCT03021486 | Active Recruiting |
Drug: Chlorpromazine Drug: Haloperidol |
Delirium Advanced Malignant Neoplasm |
M.D. Anderson Cancer Center | June 5, 2017 | Phase 2 Phase 3 |
| NCT01404364 | Completed | Drug: Triamcinolone Drug: Chlorpromazine |
Blind Painful Eye Refractory Glaucoma |
Hospital Governador Celso Ramos | January 2010 | Not Applicable |
| NCT00202293 | Completed | Drug: Olanzapine Drug: Lithium Drug: Chlorpromazine |
Bipolar Disorder Schizoaffective Disorder |
Melbourne Health | October 1, 2001 | Phase 4 |
| NCT03639558 | Completed | Drug: Haloperidol + Promethazine | Aggression Agitation |
Joseph Dib | August 28, 2018 | Phase 4 |