| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 50g |
|
||
| Other Sizes |
| 靶点 |
Plasmodium
|
|---|---|
| 体外研究 (In Vitro) |
亚甲基蓝作为替代电子受体/供体,有助于修复线粒体、增强神经能量生成并防止超氧化物形成[1]。亚甲蓝抑制细胞色素 P450 (CYP) 同工酶。当与水结合时,亚甲蓝(一种无味、水溶性、深蓝绿色结晶粉末)会变成蓝色。一种称为亚甲蓝的血管升压药会抑制诱导型 NOS,进而抑制 sGC 的激活,从而影响 NO 合成途径。亚甲基蓝还通过附着在 sGC 的铁血红素部分并直接与 NO 竞争激活可溶性鸟苷酸环化酶的能力来抑制环 GMP (cGMP) 的积累[3]。
LPS 激活的 BV2 小胶质细胞可以改变其免疫学特征,并通过亚甲蓝 (Basic Blue 9)(4.5 μM;BV2 小胶质细胞)降低其 CD14、IL-1β、TNF-α 和 CCL2 mRNA 水平 [3]。
Methylene blue (MB)/亚甲基蓝是吩噻嗪和其他衍生物的第一个先导化学结构,通常用于诊断程序和治疗高铁血红蛋白血症。我们之前已经证明,MB可以作为一种替代的线粒体电子转移载体,增强细胞耗氧量,并在体外和帕金森病和中风的啮齿动物模型中提供保护。在本研究中,我们使用MB和六种结构相关化合物研究了MB的体外构效关系。MB通过绕过线粒体复合物I-III的替代电子转移减少线粒体超氧化物的产生。MB减轻了反应性自由基的产生,并在HT-22细胞中提供了针对谷氨酸、IAA和鱼藤酮毒性的神经保护。显然,MB对葡萄糖氧化酶诱导的直接氧化应激没有保护作用。在MB的10个氮原子上替换侧链,使其对谷氨酸神经毒性的保护效力降低了1000倍。在3和7位没有侧链的化合物,氯苯噻嗪和吩噻嗪,与MB相比具有不同的氧化还原电位,不能增强线粒体电子转移,同时对谷氨酸、IAA和鱼藤酮损伤具有直接的抗氧化作用。与MB相比,氯苯噻嗪在线粒体裂解物分析中表现出直接的抗氧化作用,MB需要NADH和线粒体的还原。MB增加了复合物IV的表达和活性,而2-氯苯噻嗪没有影响。我们的研究表明,MB可以通过作为线粒体电子转移载体和线粒体中可再生的抗氧化剂来减弱超氧化物的产生[4]。 |
| 体内研究 (In Vivo) |
在雄性 Sprague-Dawley 大鼠(7 周龄,200-250 g)中,亚甲蓝(1、5 和 25 μg/大鼠)显着降低脑环磷酸鸟苷 (cGMP) 含量和七氟醚最低肺泡麻醉浓度 (MAC) )以剂量依赖性方式[2]。在染色内窥镜检查过程中,将亚甲蓝喷入胃肠道粘膜作为染料来检测不典型增生或癌前病变[2]。亚甲蓝可以使平均动脉压 (MAP) 正常化,恢复血管张力,并减少对血管加压药的需求[3]。
|
| 酶活实验 |
线粒体膜电位分析[4]
如前所述,使用TMRE/NAO通过FRET分析线粒体膜电位。TMRE在正常线粒体膜电位下淬灭NAO荧光。随着膜电位的降低,TMRE荧光降低,导致NAO荧光增加。NAO荧光的增加被解释为线粒体膜电位的降低。细胞与谷氨酸和亚甲基蓝(MB)或相关化合物一起孵育12小时。然后移除培养基,用PBS洗涤细胞一次,然后在37°C下在含有1µM NAO和1µM TMRE的PBS中孵育30分钟。移除NAO/TMRE,在KRH中于37°C下再孵育细胞15分钟。在PBS中洗涤细胞两次,使用Tecan Infinite F200平板读数器测量NAO荧光(激发485,发射530)。原始数据表示为RFU。然后根据对照和钙黄绿素AM细胞活力对NAO荧光进行标准化。 活性氧物种分析[4] 使用荧光酶标仪、流式细胞术和荧光显微镜,通过ROS反应性荧光指示剂H2DCFDA(Anaspec)测量细胞ROS的变化。对于微孔板实验,HT-22细胞以3000个细胞/孔的密度在96孔板中放置过夜。细胞与药物和20 mM谷氨酸在37°C和5%CO2下孵育12小时。然后移除培养基,用PBS洗涤细胞一次,然后在37°C下在含有10µM H2DCFDA的PBS中孵育30分钟。移除PBS,在KRH中于37°C下再孵育细胞15分钟。在PBS中洗涤细胞两次,使用帝肯Infinite F200平板读数器测量DCF荧光(激发485,发射530)。原始数据表示为RFU。然后根据对照和钙黄绿素AM细胞存活率对DCF荧光进行标准化。对于荧光显微镜,HT-22细胞以10000个细胞/孔的密度铺在6孔板上。细胞在谷氨酸和指定药物中孵育8小时。8小时后,用含有10µM H2DCFDA的KRH培养基替换培养基。细胞在37°C下孵育15分钟,用KRH洗涤一次,然后在37°C下在新鲜KRH中再孵育10分钟。用新鲜的KRH缓冲液替换培养基,并对细胞进行成像。对于流式细胞术,HT-22细胞以50000个细胞/孔的密度接种在6孔培养皿中,并附着过夜。移除培养基,用含有新鲜DMEM(高糖、1 mM丙酮酸盐、10%FBS)的载体、10µM亚甲基蓝(MB)、20 mM谷氨酸盐或10µM亚甲基蓝(MB)。细胞在37°C和5%CO2下孵育8小时。孵育后,取出培养基,用PBS洗涤细胞一次,并在37°C下在含有10µM H2DCFDA的PBS中孵育15分钟。移除PBS,在37°C的PBS中再孵育细胞10分钟。用新鲜PBS替换PBS,用Beckman Coulter FC-500测定DCF荧光。 线粒体裂解物氧化试验[4] 在含有500µM H2O2、10µM DCF的10 mM磷酸盐缓冲液(pH=7.4)中,在存在或不存在165µM NADH和线粒体裂解物(19.4µg/ml)的情况下,对四种化合物(亚甲基蓝(MB)、NR、2-氯吩噻嗪和氯丙嗪)进行了测定。在37°C下,在Greiner 96孔黑板上进行30分钟的测定,此时用Tecan Infinite F200板读数器测量DCF荧光(激发485,发射530)。 |
| 细胞实验 |
细胞活力测定[4]
通过钙黄绿素AM和MTT法测定细胞存活率。对于钙黄绿素AM测定,HT-22细胞以3000个细胞/孔的密度接种,并在100µl DMEM(含1 mM丙酮酸和10%FBS的高糖)的96孔板中孵育过夜。向每个孔中加入不同浓度的亚甲基蓝(MB)或其衍生物和20 mM谷氨酸盐,并在37°C和5%CO2下孵育12小时。12小时后,取出培养基,用1µM的钙黄绿素AM PBS溶液替换。细胞在37°C下孵育5分钟,使用帝肯Infinite F200平板读数器测量荧光(激发485发射530)。对于MTT测定,将HT-22细胞以3000个细胞/孔的密度接种到100µl DMEM(高糖、1 mM丙酮酸、10%FBS)中的96孔平底板中,并允许其附着过夜。然后向每个孔中加入不同浓度的药物和20mM谷氨酸盐(或对照孔的培养基)。在37°C和5%CO2的条件下孵育板12小时。从培养箱中取出培养板,每孔加入20µl MTT(PBS中5mg/ml)。轻轻搅拌平板以将MTT混合到培养基中,然后将其放回培养箱中2小时。2小时后,取出培养基,向每个孔中加入100µl DMSO。通过温和搅拌将板混合,并用Tecan Infinite F200板读数器测量吸光度(560nm,参考670nm)。 鱼藤酮神经毒性试验[4] 将HT-22细胞以3000个细胞/孔的密度接种到100µl DMEM(高糖、1 mM丙酮酸、10%FBS)中的96孔平底板中,并允许其附着过夜。然后向每个孔中加入不同浓度的亚甲基蓝(MB)或其衍生物和5µM鱼藤酮(或对照孔的培养基)。在37°C和5%CO2的条件下孵育板24小时。通过钙黄绿素AM测定存活率。 葡萄糖氧化酶神经毒性试验[4] 将HT-22细胞以3000个细胞/孔的密度接种到100µl DMEM(高糖、1 mM丙酮酸、10%FBS)中的96孔平底板中,并允许其附着过夜。然后向每个孔中加入不同浓度的亚甲基蓝(MB)或其衍生物和2U葡萄糖氧化酶(或对照孔的培养基)。在37°C和5%CO2的条件下孵育板3小时。通过钙黄绿素AM测定存活率。 碘乙酸(IAA)神经毒性试验[4] 将HT-22细胞以3000个细胞/孔的密度接种到100µl DMEM(高糖、1 mM丙酮酸、10%FBS)中的96孔平底板中,并允许其附着过夜。然后向每个孔中加入不同浓度的亚甲基蓝(MB)或其衍生物和20µM IAA(或对照孔的培养基)。将培养板在37°C和5%CO2的条件下孵育2小时。2小时后,取出所有培养基,用含有药物但不含IAA的新鲜培养基替换。在37°C和5%CO2的条件下,将平板再孵育22小时。通过钙黄绿素AM测定存活率。 蛋白质印迹[4] HT-22电池以150000/孔的密度镀在6孔板中。细胞附着过夜,第二天以指定浓度向细胞中加入亚甲基蓝(MB)或2-氯吩噻嗪。细胞生长3天,并在含有蛋白酶和磷酸酶抑制剂的放射免疫沉淀试验(RIPA)缓冲液中裂解。将细胞裂解物装载到10%聚丙烯酰胺凝胶上,并转移到硝化纤维上。硝化纤维与第一抗体在4°C下以指定浓度(Cox1,1∶500;肌动蛋白,1∶3000)孵育过夜。与辣根过氧化物酶连接的二抗在室温下孵育2小时(1∶2000稀释)。用UVP Biospectrum 500检测化学发光。 |
| 动物实验 |
The nitric oxide (NO)-cyclic guanosine monophosphate (cGMP) signal pathway plays an important role in anesthetic and analgesic effects. We sought to determine the involvement of inhibition of soluble guanylyl cyclase (sGC) in the anesthetic mechanism and site of action of volatile anesthetics. We examined the effect of intracerebroventricular (ICV) administration of methylene blue (MB), a sGC inhibitor, on the minimum alveolar anesthetic concentration (MAC) of sevoflurane and the brain cGMP content in rats in vivo. We also investigated the effect of sevoflurane on NO-stimulated sGC activity in vitro. The rats were divided into three groups. After the ICV administration of MB, sevoflurane MAC and brain cGMP contents were measured in the first group and the second group, respectively. In the third group, brain cGMP contents were determined after sevoflurane anesthesia without the ICV administration of MB to examine the direct effect of sevoflurane on brain cGMP contents. MB significantly decreased sevoflurane MAC and brain cGMP content in a dose-dependent manner. Sevoflurane itself also dose-dependently decreased cGMP contents in brain in vivo and inhibited the NO-stimulated sGC activity in vitro. These results suggest that the inhibition of the NO-cGMP signal pathway at the sGC level could be involved in anesthetic or analgesic effects, and the inhibitory effect of sevoflurane on sGC would be one of the sites of action of this anesthetic.
Implications: Because the nitric oxide-cyclic guanosine monophosphate signal pathway mediates nociception and the site of action of halogenated volatile anesthetics in uncertain, we examined the possible involvement of inhibition of soluble guanylyl cyclase in the anesthetic mechanism. The inhibitory effect of sevoflurane on soluble guanylyl cyclase could be one of sites of this anesthetic.[3]
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Excreted in urine and bile. About 75% of an oral dose excreted in urine, primarily as stabilized colorless leukomethylene blue. 10 mg/kg (in rats). 3.0 ± 0.7 L/min. ... The concentration of methylene blue in whole blood was measured using high-performance liquid chromatography in seven volunteers after IV and oral administration of 100 mg methylene blue with and without mesna. The distribution of methylene blue in different tissues was measured in rats after intraduodenal and IV application. The time course of methylene blue in whole blood after IV administration showed a multiphasic time course with an estimated terminal half-life of 5.25 hr. Following oral administration, the area under the concentration-time curve was much lower (9 nmol/min/mL vs 137 nmol/min/mL). Co-administration of mesna, which could influence distribution by ion-pairing, did not alter the pharmacokinetics. The urinary excretion of methylene blue and its leukoform was only moderately higher after IV administration (18% vs 28% dose). Intraduodenal administration to rats resulted in higher concentrations in intestinal wall and liver but lower concentrations in whole blood and brain than IV methylene blue. Differences in organ distribution of methylene blue are mainly responsible for the different pharmacokinetics after oral and IV administration. ... Methylene blue is well absorbed from the GI tract, and peak plasma concentrations occur approximately 1-2 hours after an oral dose. ... Following distribution into tissues, methylene blue is rapidly reduced to leukomethylene blue (leucomethylthioninium chloride). Metabolism to leucomethylene blue may be less efficient in neonates than in older individuals. Methylene blue is excreted in urine and bile. About 75% of an oral dose of methylene blue is excreted in urine, mostly as stabilized colorless leukomethylene blue. On exposure to air, the urine turns green or blue, due to the presence of the oxidation product methylene azure (methylene blue sulfone). Some unchanged drug is also excreted in urine. BACKGROUND: Although blue dye is routinely used for lymphatic mapping, it is not used for lymphatic mapping in pregnancy-associated breast cancer, because of concern of fetal risk. METHODS: To investigate the safety of blue dye for lymphatic mapping in pregnant women, the pharmacokinetics of methylene blue dye were examined in 10 nonpregnant women, and the results were extrapolated to estimate maximal fetal exposure to the dye. RESULTS: Plasma and urine measurements indicated that the dye quickly distributed from the breast injection site to the circulation, with 32% of the total dose excreted in urine within 48 hours. Combined with existing data on organ distribution of methylene blue, the estimated maximal dose to the fetus is 0.25 mg (5% of the administered dose), likely further reduced by other physiologic factors related to pregnancy. CONCLUSIONS: The analysis suggests that methylene blue dye can be used for lymphatic mapping in pregnancy-associated breast cancer with minimal fetal risk. The disposition and urinary excretion pharmacokinetics of methylene blue were determined after its intravenous administration at 15 mg/kg to mature female sheep. Comparisons were made between methylene blue administered alone or subsequent to 50 mg/kg sodium nitrite. The overall elimination rate constant (beta) of methylene blue, 0.0076 +/- 0.0016 min-1, was not influenced by prior administration of sodium nitrite. However, the distribution rate was significantly altered by sodium nitrite. Very little of the methylene blue was eliminated in the urine either intact or as leukomethylene blue in spite of its relatively short half life. ... Metabolism / Metabolites Following distribution into tissues, rapidly reduced to leukomethylene blue (leucomethylthioninium chloride). Metabolism to leucomethylene blue may be less efficient in neonates than in older individuals. Methylene blue can be reduced to a colorless form, leukomethylene blue; together, these compounds form a reversible oxidation-reduction system. In low concentrations, methylene blue accelerates conversion of methemoglobin to hemoglobin. In patients with methemoglobinemia, methylene blue is reduced to leukomethylene blue by methemoglobin reductases in erythrocytes; leukomethylene blue then reduces methemoglobin to hemoglobin. In high concentrations, methylene blue oxidizes the ferrous iron of reduced hemoglobin to the ferric state, thereby changing hemoglobin to methemoglobin. Following distribution into tissues, methylene blue is rapidly reduced to leukomethylene blue (leucomethylthioninium chloride). Metabolism to leucomethylene blue may be less efficient in neonates than in older individuals. Biological Half-Life 5–6.5 hours (after IV dose). Following IV administration, the estimated half-life of methylene blue is 5-6.5 hours. ... The concentration of methylene blue in whole blood was measured using high-performance liquid chromatography in seven volunteers after IV and oral administration of 100 mg methylene blue with and without mesna. The distribution of methylene blue in different tissues was measured in rats after intraduodenal and IV application. The time course of methylene blue in whole blood after IV administration showed a multiphasic time course with an estimated terminal half-life of 5.25 hr. ... |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Methylene blue was reported to bind strongly to rabbit plasma (71–77% of bound drug). IDENTIFICATION AND USE: Methylene blue is a solid. Water solutions are deep blue. It is used as a stain in bacteriology, as mixed indicator, a dye, a redox colorimetric agent, and a targeting agent for melanoma. It is also used as a medication to treat drug-induced methemoglobinemia. HUMAN STUDIES: Methemoglobinemia is usually treated with methylene blue. However, in patients with glucose-6-phosphate dehydrogenase deficiency, methylene blue can induce methemoglobinemia. Preclinical studies have shown that low-dose methylene blue increases mitochondrial cytochrome oxidase activity in the brain and improves memory retention after learning tasks, including fear extinction. The intracameral use of 1% methylene blue has a cytotoxic effect on the corneal endothelium and iris epithelium. Several cases of suspected serotonin syndrome have been reported in patients who received methylene blue in combination with serotonin active agents. Allergic hypersensitivity reaction to methylene blue-treated plasma transfusion has also been reported. A preterm infant had methemoglobulinemia and hemolytic anemia after enteral administration of methylene blue. There is epidemiologic evidence that methylene blue is a teratogen, and the drug can cause fetal harm if administered during pregnancy. Use of methylene blue in amniocentesis has been associated with atresia of the ileum and jejunum, ileal occlusion, and other adverse effects in neonates. Use of methylene blue during pregnancy has resulted in hemolytic anemia, hyperbilirubinemia, methemoglobinemia, respiratory distress, skin staining, and phototoxicity in neonates. ANIMAL STUDIES: Methylene blue treatment resulted in methemoglobin formation and oxidative damage to red blood cells, leading to a regenerative anemia and a variety of tissue and biochemical changes secondary to erythrocyte injury. An early change was a dose-related increase in methemoglobin, where the response of rats and mice was similar in magnitude. Mice appeared to be more sensitive than rats to the formation of Heinz bodies and the development of anemia that was characterized by a decrease in hemoglobin, hematocrit, and erythrocyte count. Splenomegaly was apparent in all treated mice and in the 100 mg/kg (males only) and 200 mg/kg rats at necropsy. Methylene blue was embryotoxic in the rat. Methylene blue caused the mice to deliver before gestation day 18 (term gestation). This response was observed in 45%, 50% and 83% of animals receiving methylene blue at 50, 60 or 85 mg/kg, respectively. Under cell-free conditions methylene blue induced DNA damage. It is characterized by a high number of base modifications sensitive to the repair endonuclease FPG protein (formamidopyrimidine-DNA glycosylase). Methylene blue was mutagenic in cultured mammalian cells. In contrast, results from the mouse micronucleus assay suggest that the genotoxicity is not expressed in vivo. The greatest concerns with methylene blue therapy in veterinary use are the development of Heinz body anemia or other red cell morphological changes, methemoglobinemia, and decreased red cell life spans. Cats tent to be very sensitive to these effects and some consider it contraindicated in feline patients, but dogs and horses can also develop hematologic adverse effects at relatively low dosages. ECOTOXICITY STUDIES: Methylene blue had a teratogenic effect in angelfish. Non-Human Toxicity Values LD50 Mouse iv 77 mg/kg LD50 Mouse ip 150 mg/kg LD50 Mouse oral 3500 mg/kg LD50 Rat iv 1250 mg/kg |
| 参考文献 | |
| 其他信息 |
Methylene blue trihydrate appears as odorless or almost odorless dark green crystals with bronze luster or dark green crystalline powder. pH (1% solution in water) 3 to 4.5. (NTP, 1992)
Methylene Blue is a synthetic basic dye. Methylene blue stains to negatively charged cell components like nucleic acids; when administered in the lymphatic bed of a tumor during oncologic surgery, methylene blue may stain lymph nodes draining from the tumor, thereby aiding in the visual localization of tumor sentinel lymph nodes. When administered intravenously in low doses, this agent may convert methemoglobin to hemoglobin. A compound consisting of dark green crystals or crystalline powder, having a bronze-like luster. Solutions in water or alcohol have a deep blue color. Methylene blue is used as a bacteriologic stain and as an indicator. It inhibits GUANYLATE CYCLASE, and has been used to treat cyanide poisoning and to lower levels of METHEMOGLOBIN. Methylene blue is an organic chloride salt having 3,7-bis(dimethylamino)phenothiazin-5-ium as the counterion. A commonly used dye that also exhibits antioxidant, antimalarial, antidepressant and cardioprotective properties. It has a role as an EC 1.4.3.4 (monoamine oxidase) inhibitor, an acid-base indicator, a fluorochrome, an antidepressant, a cardioprotective agent, an EC 3.1.1.8 (cholinesterase) inhibitor, a histological dye, an EC 4.6.1.2 (guanylate cyclase) inhibitor, an antioxidant, an antimicrobial agent, a neuroprotective agent, a physical tracer and an antimalarial. It contains a 3,7-bis(dimethylamino)phenothiazin-5-ium. Methylene blue is an oxidation-reduction agent. The intravenous form of methylene blue is approved by the FDA for the treatment of pediatric and adult patients with acquired methemoglobinemia. Historically, it has been widely used in Africa to treat malaria, but now it disappeared when chloroquine (CQ) and other drugs entered the market. Its use as an urinary tract antiseptic has also been investigated. Methylthioninium chloride (INN, or methylene blue, proposed trade name Rember) is an investigational drug being developed by the University of Aberdeen and TauRx Therapeutics that has been shown in early clinical trials to be an inhibitor of Tau protein aggregation. The drug is of potential interest for the treatment of patients with Alzheimer's disease. Methylene Blue is a synthetic basic dye. Methylene blue stains to negatively charged cell components like nucleic acids; when administered in the lymphatic bed of a tumor during oncologic surgery, methylene blue may stain lymph nodes draining from the tumor, thereby aiding in the visual localization of tumor sentinel lymph nodes. When administered intravenously in low doses, this agent may convert methemoglobin to hemoglobin. A compound consisting of dark green crystals or crystalline powder, having a bronze-like luster. Solutions in water or alcohol have a deep blue color. Methylene blue is used as a bacteriologic stain and as an indicator. It inhibits GUANYLATE CYCLASE, and has been used to treat cyanide poisoning and to lower levels of METHEMOGLOBIN. Drug Indication Indicated for the treatment of pediatric and adult patients with acquired methemoglobinemia. Other clinical applications of methylene blue include improvement of hypotension associated with various clinical states, an antiseptic in urinary tract infections, treatment of hypoxia and hyperdynamic circulation in cirrhosis of liver and severe hepatopulmonary syndrome, and treatment of ifofosamide induced neurotoxicity. Lumeblue is indicated as a diagnostic agent enhancing visualisation of colorectal lesions in adult patients undergoing screening or surveillance colonoscopy. Acute symptomatic treatment of medicinal and chemical products- induced methaemoglobinaemia. Methylthioninium chloride Proveblue is indicated in adults, children and adolescents (aged 0 to 17 years old). Mechanism of Action * Main mechanism of action involves inhibition of nitric oxide synthase and guanylate cyclase. * In Alzheimers Disease: a mechanistic study found that methylene blue oxidizes cysteine sulfhydryl groups on tau to keep tau monomeric. One preclinical treatment study in tauopathy mice reported anti-inflammatory or neuroprotective effects mediated by the Nrf2/antioxidant response element (ARE); another reported insoluble tau reduction and a learning and memory benefit when given early. * In Methemoglobinemia: Methylene Blue acts by reacting within RBC to form leukomethylene blue, which is a reducing agent of oxidized hemoglobin converting the ferric ion (fe+++) back to its oxygen-carrying ferrous state(fe++). * As antimalarial agent: Methylene Blue, a specific inhibitor of P.falciparum glutathione reductase has the potential to reverse CQ resistance and it prevents the polymerization of haem into haemozoin similar to 4-amino-quinoline antimalarials. * For ifosfamide induced neurotoxicity: Methylene blue functions as an alternate electron acceptor. It acts to reverse the NADH inhibition caused by gluconeogenesis in the liver while blocking the transformation of chloroethylamine into chloroacetaldehyde. In addition, it inhibits various amine oxidase activities, which also prevents the formation of chloroacetaldehyde. The mechanism of modulation of cyclic guanosine monophosphate accumulation by methylene blue, a putative inhibitor of soluble guanylate cyclase, was investigated in cultured rabbit pulmonary arterial smooth muscle cells. Control or methylene blue pretreated rabbit pulmonary arterial smooth muscle were stimulated with sodium nitroprusside, nitrosothiols or endothelium derived relaxing factor released basally from bovine pulmonary arterial endothelial cells, in short term cocultures. The putative endothelium-derived relaxing factor, S-nitroso-L-cysteine, a stab1e deaminated analog of S-nitroso-L-cysteine, S-nitroso-3-mercaptoproprionic acid and sodium nitroprusside produced concentration-dependent (1-100 uM) increase (1.5- to 12-fold) in rabbit pulmonary arterial smooth muscle cells cyclic guanosine monophospate levels. Methylene blue pretreatment inhibited S-nitroso-L-cysteine and sodium nitroprusside induced cyclic guanosine monophosphate accumulation by 51% to 100%, but S-nitroso-3-mercaptoproprionic acid mediated responses were not altered by methylene blue. The inhibition profile of methylene blue on nitrovasodilator induced cyclic guanosine monophosphate accumulation was quantitatively reproduced by extracellular generation of superoxide anion with xanthine (100 uM) and xanthine oxidase (5 mU). Similarly to methylene blue pretreatment, superoxide anion generation had no effects on base-line cyclic guanosine phosphate levels or cyclic guanosine phosphate responses elicited by S-nitroso-3-mercaptoproprionic acid. Furthermore, methylene blue induced a dose and time dependent generation of superoxide anion from rabbit pulmonary arterial smooth muscle cells, as evidenced from spectrophotometric determination of cytochrome c reduction. Inhibition of cyclic guanosine monophosphate accumulation in response to S-nitroso-L-cysteine and sodium nitroprusside by methylene blue was completely prevented by superoxide dismutase but not catalase. Selective pretreatment of endothelial cells with methylene blue before co-culture with untreated rabbit pulmonary arterial smooth muscle produced a reduction in rabbit pulmonary arterial smooth muscle cyclic guanosine monophosphate levels of a magnitude comparable with that seen in cocultures of methylene blue pretreated rabbit pulmonary arterial smooth muscle with untreated endothelial cells, and which was partially prevented by superoxide dismutase. Schizophrenia is a major public health problem that affects approximately 1% of the population worldwide. Schizophrenia-like symptoms can be induced in humans by phencyclidine (PCP), a drug with marked psychotomimetic properties. Phencyclidine disrupts prepulse inhibition of acoustic startle in rodents, a measure which has also been shown to be disrupted in schizophrenic patients. This effect is blocked by nitric oxide synthase (NOS) inhibitors, suggesting that nitric oxide plays an important role in this effect of phencyclidine. Methylene blue, a guanylate cyclase and nitric oxide syntase inhibitor, has shown therapeutic value as an adjuvant to conventional antipsychotics in the therapy of schizophrenia. The aim of the present study was to investigate if phencyclidine-(4 mg/kg)induced disruption of prepulse inhibition could be affected by methylene blue (50 or 100 mg/kg) in mice. Furthermore, the effect of methylene blue (50 mg/kg) on phencyclidine-(4 mg/kg)induced hyperlocomotion was investigated. The present study shows that phencyclidine readily disrupts prepulse inhibition in mice without affecting pulse-alone trials. It was also found that methylene blue prevents the decrease in prepulse inhibition caused by phencyclidine in a dose-related manner. Furthermore, the increase in locomotor activity caused by phencyclidine was reduced by pretreatment with methylene blue. The results from the present study further support the suggestion that the nitric oxide synthase/guanylate cyclase pathway is involved in pharmacological and behavioural effects of phencyclidine. Since phencyclidine as well exerts psychotomimetic characteristics, agents that interfere with the nitric oxide synthase/guanylate cyclase pathway may be of therapeutic value also in the treatment of schizophrenia.[1] Introduction: Neurofibrillary tangles (NFT) composed of Tau are hallmarks of neurodegeneration in Alzheimer disease. Transgenic mice expressing full-length pro-aggregant human Tau (2N4R Tau-ΔK280, termed Tau(ΔK)) or its repeat domain (TauRD-ΔK280, TauRD(ΔK)) develop a progressive Tau pathology with missorting, phosphorylation, aggregation of Tau, loss of synapses and functional deficits. Whereas TauRD(ΔK) assembles into NFT concomitant with neuronal death, Tau(ΔK) accumulates into Tau pretangles without overt neuronal loss. Both forms cause a comparable cognitive decline (with onset at 10mo and 12mo, respectively), which is rescued upon switch-off of transgene expression. Since methylene blue (MB) is able to inhibit Tau aggregation in vitro, we investigated whether MB can prevent or rescue Tau-induced cognitive impairments in our mouse models. Both types of mice received MB orally using different preventive and therapeutic treatment protocols, initiated either before or after disease onset. The cognitive status of the mice was assessed by behavior tasks (open field, Morris water maze) to determine the most successful conditions for therapeutic intervention. Results: Preventive and therapeutic MB application failed to avert or recover learning and memory deficits of TauRD(ΔK) mice. Similarly, therapeutic MB treatment initiated after onset of cognitive impairments was ineffective in Tau(ΔK) mice. In contrast, preventive MB application starting before onset of functional deficits preserved cognition of Tau(ΔK) mice. Beside improved learning and memory, MB-treated Tau(ΔK) mice showed a strong decrease of insoluble Tau, a reduction of conformationally changed (MC1) and phosphorylated Tau species (AT180, PHF1) as well as an upregulation of protein degradation systems (autophagy and proteasome). This argues for additional pleiotropic effects of MB beyond its properties as Tau aggregation inhibitor. Conclusions: Our data support the use of Tau aggregation inhibitors as potential drugs for the treatment of AD and other tauopathies and highlights the need for preventive treatment before onset of cognitive impairments.[2] Traumatic brain injury (TBI) is associated with cerebral edema, blood brain barrier breakdown, and neuroinflammation that contribute to the degree of injury severity and functional recovery. Unfortunately, there are no effective proactive treatments for limiting immediate or long-term consequences of TBI. Therefore, the objective of this study was to determine the efficacy of methylene blue (MB), an antioxidant agent, in reducing inflammation and behavioral complications associated with a diffuse brain injury. Here we show that immediate MB infusion (intravenous; 15-30 minutes after TBI) reduced cerebral edema, attenuated microglial activation and reduced neuroinflammation, and improved behavioral recovery after midline fluid percussion injury in mice. Specifically, TBI-associated edema and inflammatory gene expression in the hippocampus were significantly reduced by MB at 1 d post injury. Moreover, MB intervention attenuated TBI-induced inflammatory gene expression (interleukin [IL]-1β, tumor necrosis factor α) in enriched microglia/macrophages 1 d post injury. Cell culture experiments with lipopolysaccharide-activated BV2 microglia confirmed that MB treatment directly reduced IL-1β and increased IL-10 messenger ribonucleic acid in microglia. Last, functional recovery and depressive-like behavior were assessed up to one week after TBI. MB intervention did not prevent TBI-induced reductions in body weight or motor coordination 1-7 d post injury. Nonetheless, MB attenuated the development of acute depressive-like behavior at 7 d post injury. Taken together, immediate intervention with MB was effective in reducing neuroinflammation and improving behavioral recovery after diffuse brain injury. Thus, MB intervention may reduce life-threatening complications of TBI, including edema and neuroinflammation, and protect against the development of neuropsychiatric complications.[3] |
| 分子式 |
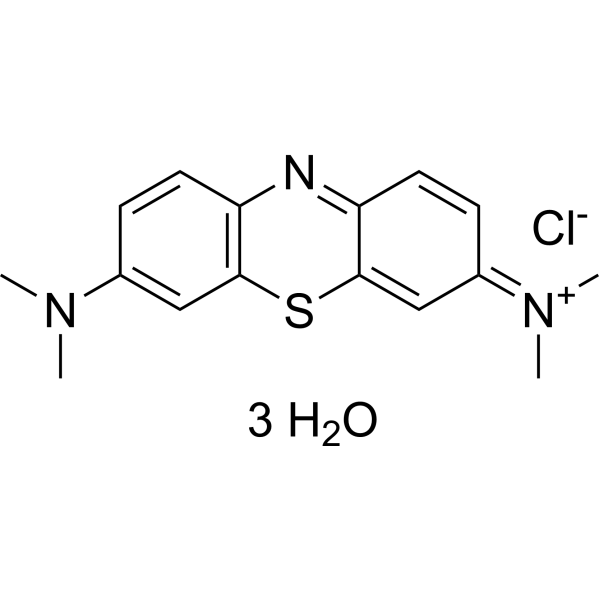
C16H24CLN3O3S
|
|---|---|
| 分子量 |
373.90
|
| 精确质量 |
373.122
|
| 元素分析 |
C, 51.40; H, 6.47; Cl, 9.48; N, 11.24; O, 12.84; S, 8.57
|
| CAS号 |
7220-79-3
|
| 相关CAS号 |
Methylene blue trihydrate;7220-79-3;Methylene blue hydrate;122965-43-9; 61-73-4 (Cl)
|
| PubChem CID |
104827
|
| 外观&性状 |
Light brown to black solid powder
|
| 密度 |
0.98 g/mL at 25 °C
|
| 熔点 |
190 °C (dec.)(lit.)
|
| 闪点 |
14 °C
|
| tPSA |
75.07
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
483
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
XQAXGZLFSSPBMK-UHFFFAOYSA-M
|
| InChi Code |
InChI=1S/C16H18N3S.ClH.3H2O/c1-18(2)11-5-7-13-15(9-11)20-16-10-12(19(3)4)6-8-14(16)17-13;;;;/h5-10H,1-4H3;1H;3*1H2/q+1;;;;/p-1
|
| 化学名 |
[7-(dimethylamino)phenothiazin-3-ylidene]-dimethylazanium;chloride;trihydrate
|
| 别名 |
Methylene Blue trihydrate; 7220-79-3; Phenothiazin-5-ium, 3,7-bis(dimethylamino)-, chloride, trihydrate; C.I. Basic Blue 9 trihydrate; Methylthionine chloride; Basic Blue 9 trihydrate; 3,7-Bis(dimethylamino)phenothiazin-5-ium chloride trihydrate; C.I. Basic Blue 9, trihydrate;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 100 mg/mL (267.45 mM)
|
|---|
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6745 mL | 13.3726 mL | 26.7451 mL | |
| 5 mM | 0.5349 mL | 2.6745 mL | 5.3490 mL | |
| 10 mM | 0.2675 mL | 1.3373 mL | 2.6745 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。