| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |

| 靶点 |
Neuraminidase; influenza A/H3N2, A/H1N2, A/H1N1, and B viruses
|
|---|---|
| 体外研究 (In Vitro) |
正在考虑将奥司他韦和帕拉米韦联合治疗人类严重流感病毒感染。这两种化合物都是流感病毒神经氨酸酶抑制剂,由于帕拉米韦与酶的结合比羧酸奥司他韦(奥司他韦的活性形式)更紧密,因此两种化合物一起使用时可能会产生拮抗相互作用。为了研究这种可能性,在感染甲型流感/NWS/33 (H1N1)病毒的小鼠和体外进行了联合化疗实验。用羧酸奥司他韦和帕拉米韦在0.32-100μM浓度下联合处理感染的MDCK细胞3天,然后测定病毒产量。使用MacSynergy方法发现具有狭窄协同作用区域的加性药物相互作用。在0.01-10nM的病毒神经氨酸酶抑制剂联合试验中,在整个浓度范围内没有观察到显著的拮抗或协同作用[5]。
羧酸奥司他韦和帕拉米韦联合使用0.32至100 μM剂量对MDCK细胞培养的病毒产量进行了评估(表1)。在100 μM剂量下,羧酸奥司他韦单独使用可使病毒产量降低4.4 log10。Peramivir在32 μM和100 μM浓度下使病毒产率低于检测限≥5 log10。当10 μM羧酸奥司他韦与3.2 μM帕拉米韦和10 μM帕拉米韦联合使用,以及每种3.2 μM抑制剂联合使用时,三种特定条件下的病毒滴度均达到预期的10倍以上。数据的三维MacSynergy图显示了高于和低于预期的值,如图1所示。1 ~ 10 μM奥司他韦和1 ~ 10 μM帕拉米韦之间存在显著协同作用,协同量为9.1。当0.32 μM peramivir与3.2 ~ 32 μM oseltamivir carboxylate联合使用时,出现了一个较小的拮抗区域,计算拮抗量为- 1.7。整个地表的净效应是7.4的协同量。[5] 羧酸奥司他韦和帕拉米韦联合使用对神经氨酸酶活性的影响见表2。在10 nM奥司他韦羧酸盐处理或1 ~ 10 nM帕拉米韦处理下,神经氨酸酶活性明显降低。大多数低剂量组合(0.01至3.2 nM奥司他韦羧酸盐与0.01至0.32 nM帕拉米韦联合使用)比单独使用任何一种化合物产生更大的抑制作用。每种抑制剂联合使用的浓度较高(0.32至10 nM)引起的抑制作用比预期的要小。这是在一个区域,帕拉米韦单独是高度抑制酶活性,没有太大的潜力进一步抑制药物组合。数据的三维MacSynergy图如图2所示。这些组合增加或减少的百分比很小。低剂量联合区域的协同作用体积为86(中等协同作用),而高剂量联合区域的拮抗作用体积为- 65(中等拮抗作用),整个表面的净效应为21(无差异)。[5] |
| 体内研究 (In Vivo) |
在感染 H1N1pdm 病毒的小鼠中,口服奥司他韦(20 mg/kg/天),每日两次,持续 10 天,可以预防死亡[2]。在感染H5N1流感病毒的小鼠中,奥司他韦(10 mg/kg/天,口服灌胃,5天)联合金刚烷胺(HY-B0402)(15或30 mg/kg)比单药治疗具有更有效的保护作用[3 ]。为了阻止小鼠感染流感病毒,奥司他韦还可以与利巴韦林 (HY-B0434)、法匹拉韦 (HY-14768) 或帕拉米韦 (HY-17015A) 联合给药 [4][5]。
从病毒攻毒前2小时开始,每天给感染小鼠治疗2次,连续5天,剂量为0.05-0.4mg/kg/天。与单一药物治疗相比,每日两次口服奥司他韦(0.4mg/kg/天)与每日两次肌肉注射帕拉米韦(0.1和0.2mg/kg/天)联合使用时,幸存者人数出现了一致且具有统计学意义的增加。数据表明,在治疗小鼠流感感染方面,奥司他韦和帕拉米韦联合使用比单独使用每种化合物的次优剂量效果更好。应考虑用这两种化合物治疗。[5] 金刚烷胺(15或30毫克/公斤/天)和奥司他韦(10毫克/公斤/天)联合治疗对金刚烷胺敏感的H5N1病毒致命感染提供了比单一治疗更高的保护(分别为60%和90%)。此外,两种联合疗法都阻止了病毒向大脑的传播。联合用药对金刚烷胺耐药H5N1病毒的疗效与单独使用奥司他韦相当。奥司他韦对两种重组H5N1病毒均产生剂量依赖效应(P < 0.05),但对致命感染不提供完全保护。重要的是,当两种药物联合使用时,没有检测到HA, NA和M2蛋白的突变。结论:与单药治疗相比,联合化疗对接种嗜神经型H5N1流感病毒的小鼠具有生存优势。这种策略可能是控制对金刚烷胺敏感的大流行性流感病毒的一种选择。应探索包括其他药物的联合用药。[3] 我们研究了一种神经氨酸酶抑制剂(奥司他韦)和一种流感病毒聚合酶抑制剂(利巴韦林)对两种高致病性H5N1流感病毒的作用。在体外,A/Vietnam/1203/04病毒(进化支系1)对羧酸奥司他韦高度敏感(50%抑制浓度[IC(50)] = 0.3 nM),而A/Turkey/15/06病毒(进化支系2.2)对羧酸奥司他韦的敏感性较低(IC(50) = 5.5 nM)。在体内,BALB/c小鼠用奥司他韦(1、10、50或100 mg/kg体重/天)、利巴韦林(37.5、55或75 mg/kg/天)或两种药物联合治疗8天,从病毒接种前4小时开始。单药治疗在体内对两种H5N1病毒产生剂量依赖性抗病毒作用。药物-药物相互作用的三维分析表明,奥司他韦和利巴韦林主要以加性方式相互作用,在某些浓度下有几种边际协同作用或边际拮抗作用。利巴韦林37.5 mg/kg/d与奥司他韦1 mg/kg/d联用和利巴韦林37.5 mg/kg/d与奥司他韦10 mg/kg/d联用对A/Vietnam/1203/04和A/Turkey/15/06病毒均有增效作用。这些最佳的奥司他韦-利巴韦林组合显著抑制了病毒在小鼠器官中的复制,阻止了H5N1病毒在呼吸道以外的传播,并消除了细胞因子反应(P < 0.01)。重要的是,我们观察到两种药物组合对两种H5N1病毒的疗效之间存在明显差异:A/Turkey/15/06病毒对小鼠的保护剂量要高于A/Vietnam/1203/04病毒对小鼠的保护剂量。我们的初步结果表明,奥司他韦-利巴韦林联合治疗比单药治疗具有更强或更弱的抗病毒效果,这取决于H5N1病毒和使用的浓度。[4] |
| 酶活实验 |
病毒神经氨酸酶抑制试验[5]
化合物对病毒神经氨酸酶活性的影响使用市售试剂盒(NA-Star®流感神经氨酸酶抑制剂耐药性检测试剂盒,应用生物系统公司,加利福尼亚州福斯特市),按照制造商的说明,在96孔固体白色微孔板中测定,并已报道(Smee等,2010)。以半对数稀释增量的化合物与病毒(作为神经氨酸酶的来源)孵育。每个微孔中流感病毒A/NWS/33 (H1N1)的数量约为500个细胞培养感染剂量。在加入化学发光底物之前,在37℃下预孵育10分钟。加入底物后,在37℃下孵育30分钟。在加入NA-Star®加速剂溶液后立即使用Centro LB 960光度计(Berthold Technologies, Oak Ridge, TN)评估神经氨酸酶活性0.5秒。每种化合物浓度下化学发光计数的百分比基于未处理条件下归一化到100%的计数。 NA酶抑制试验[4] NA活性采用Potier等人描述的方法测定。简单地说,将H5N1病毒与不同浓度的羧酸奥司他韦或扎那米韦在37℃下预孵育30分钟,然后加入底物2 ' -(4-甲基伞形叶酰基)-α- d - n -乙酰神经氨酸。1 h后,加入14 mM NaOH终止反应,用激发波长360 nm,发射波长448 nm的perkins - elmer荧光仪(LS50B型)定量荧光。50%抑制浓度(IC50)定义为相对于含有病毒但不含抑制剂的反应混合物,使NA活性降低50%所需的NA抑制剂浓度。 |
| 细胞实验 |
细胞培养抗病毒研究[5]
在MDCK细胞的混合培养中测定了羧酸奥司他韦和帕拉米韦的抗病毒活性。实验在96孔微孔板上进行,感染了大约50%的细胞培养感染剂量(CCID50)的病毒,通过定量培养3天后的病毒产量。样品板在- 80℃冷冻。随后将两个微孔中的培养基混合,用于生产滴定样品。采用终点稀释法(Reed and Muench, 1938),每次稀释4个微孔,在96孔微孔板的新鲜单层MDCK细胞上滴定样品(稀释倍数为10倍),以确定每种抑制剂浓度下的病毒产量。在感染后3天和6天检测微孔板是否存在病毒细胞病理学。病毒滴度表示为log10 CCID50 / 0.1 ml。 |
| 动物实验 |
Animal experiment design[5]
Female BALB/c mice (18-20 g) were anesthetized by i.p. injection of ketamine (100 mg/kg) followed by intranasal infection with a 50-μl suspension of influenza virus; the infection inoculation of approximately 104.5 CCID50/mouse equaled three 50% mouse lethal challenge doses (MLD50). Compounds were administered p.o. (oseltamivir) by gavage or i.m. (peramivir) twice a day at 12-hour intervals for 5 days starting 2 hours before virus challenge. Placebo-treated mice received both p.o. and i.m. treatments. Ten drug-treated infected mice and 10 placebo-treated controls were observed daily for death through 21 days. Mice that died during the treatment phase were excluded from the total count. Body weights were determined every other day. In this report, we extend the in vivo observations by comparing the efficacies of JNJ63623872 and oseltamivir in mice infected with influenza A/California/04/2009 (H1N1pdm) and A/Victoria/3/75 (H3N2) viruses. Animals received JNJ63623872 or oseltamivir orally twice daily for 10 days starting 2 h pre-infection. JNJ63623872 (2, 6, and 20 mg/kg/day) and oseltamivir (20 mg/kg/day) completely prevented death in the H1N1pdm virus infection. Weight loss at nadir was only 12% in mice receiving 2 mg/kg/day of JNJ63623872 compared to 23% and 32%, respectively, in oseltamivir-treated (20 mg/kg/day) and placebo groups. Lung hemorrhage scores, lung weights, and lung virus titers on day 6 were reduced in a dose-responsive manner by JNJ63623872 treatments, whereas oseltamivir treatments were not as effective. JNJ63623872 was less active against H3N2 virus infection, with more body weight loss occurring and only 30% survival at the 2-mg/kg/day dose. Lung scores, lung weights, and H3N2 viral titers in lungs of mice were reduced less by JNJ63623872 treatments compared to the H1N1pdm infection. Nevertheless, the 20-mg/kg/day dose of JNJ63623872 was more effective than oseltamivir (20 mg/kg/day) in improving body weight and reducing the severity of lung infection. JNJ63623872 appears to be an important new drug candidate to treat influenza A H1N1pdm and H3N2 virus infections.[2] BALB/c mice were treated by oral gavage for 5 days with amantadine (1.5, 15 or 30 mg/kg/day) and oseltamivir (1 or 10 mg/kg/day) separately or in combination. Mice were challenged 24 h after initiation of treatment with 10 mouse 50% lethal doses of either amantadine-sensitive (having S31 in the M2 protein) or amantadine-resistant (having N31 in the M2 protein) recombinant A/Vietnam/1203/04 (H5N1) virus.[3] Assessment of drug efficacy in vivo.[4] Female 6-week-old BALB/c mice were anesthetized with isoflurane and were intranasally inoculated with 50 μl of 10-fold serial dilutions of A/Vietnam/1203/04 (H5N1) or A/Turkey/15/06 (H5N1) virus in phosphate-buffered saline (PBS). The 50% mouse lethal dose (MLD50) was calculated after a 21-day observation period. For the A/Vietnam/1203/04 (H5N1) and the A/Turkey/15/06 (H5N1) viruses, the MLD50s/ml were ∼1 PFU and 4 PFU, respectively. Groups of 15 mice each were then given oseltamivir (1, 10, 50, or 100 mg/kg of body weight/day) or ribavirin (37.5, 55, or 75 mg/kg/day) by oral gavage twice daily for 8 days. In the combination treatment experiments, oseltamivir was coadministered with ribavirin on the same schedule. Virus-inoculated control mice received sterile PBS (placebo). The first drug dose was given 4 h before intranasal inoculation with 5 MLD50/mouse of A/Vietnam/1203/04 (H5N1) or 5 MLD50/mouse of A/Turkey/15/06 (H5N1) virus; these doses were equivalent to ∼4 and 20 PFU/mouse, respectively. Survival and weight change were observed; animals that showed signs of severe disease and weight loss of >25% were humanely killed. Three mice each in the experimental and the placebo groups were killed on day 3 after inoculation; and the lungs, brains, and spleens were removed, homogenized, and suspended in 1 ml of PBS. Virus from each organ was titrated by inoculation of embryonated chicken eggs with serial dilutions of the suspensions. The titers were calculated by the method of Reed and Muench (32) and are expressed as the mean log10 50% egg-infective dose (EID50)/ml ± standard deviation (SD). The limit of virus detection was 0.75 log10 EID50/ml. For calculation of the mean, samples with a virus titer of <0.75 log10 EID50/ml were assigned a value of 0.[4] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Oseltamivir is readily absorbed from the gastrointestinal tract after oral administration of oseltamivir phosphate and is extensively converted by predominantly hepatic esterases to the active metabolite oseltamivir carboxylate. At least 75 % of an oral dose reaches the systemic circulation as the active metabolite. Exposure to the pro-drug is less than 5 % relative to the active metabolite. Plasma concentrations of both pro-drug and active metabolite are proportional to dose and are unaffected by co-administration with food. Pharmacokinetic parameters following twice daily dosing of oseltamivir 75mg capsules are as follows: Cmax of oseltamivir and oseltamivir carboxylate were found to be 65ng/mL and 348ng/mL, respectively, while AUC (0-12h) of oseltamivir and oseltamivir carboxylate were found to be 112ng·h/mL and 2719ng·h/mL, respectively. Following absorption, oseltamivir is more than 90 % eliminated through conversion to oseltamivir carboxylate and subsequent elimination entirely through renal excretion. During clinical studies, less than 20 % of oral radiolabelled dose was found to be eliminated in faeces. The mean volume of distribution at steady state of the oseltamivir carboxylate ranges approximately between 23 and 26 liters in humans, a volume that is roughly equivalent to extracellular body fluid. Since neuraminidase activity is extracellular, oseltamivir carboxylate distributes to all sites of influenza virus spread. Renal clearance (18.8 l/h) of the drug exceeds glomerular filtration rate (7.5 l/h) indicating that tubular secretion occurs in addition to glomerular filtration. Protein binding: Oseltamivir phosphate: Moderate (42%). Oseltamivir carboxylate: Very low < 3%. Oseltamivir carboxylate: Volume of distribution is 23 to 26 liters following intravenous administration in 24 subjects. Oral oseltamivir phosphate is readily absorbed then extensively converted to oseltamivir carboxylate, the active form, predominantly by hepatic esterases. At least 75% of an oral dose reaches the systemic circulation as oseltamivir carboxylate. Less than 5% of an oral dose reaches the systemic circulation as oseltamivir phosphate. Elimination: Renal: Oseltamivir carboxylate is extensively eliminated by renal excretion (> 99%). Renal clearance (18.8 L/hr) exceeds glomerular filtration rate (7.5 L/hr), indicating that tubular secretion occurs. Fecal: Elimination of an oral radiolabeled dose in < 20% in the feces. For more Absorption, Distribution and Excretion (Complete) data for OSELTAMIVIR (8 total), please visit the HSDB record page. Metabolism / Metabolites Oseltamivir is extensively converted to the active metabolite, oseltamivir carboxylate, by esterases located predominantly in the liver. Oseltamivir carboxylate is not further metabolized. Neither oseltamivir nor oseltamivir carboxylate is a substrate for, or inhibitor of, cytochrome P450 isoforms. No phase 2 conjugates of either compound have been identified in vivo. Oseltamivir is extensively converted to oseltamivir carboxylate by esterases located predominantly in the liver. Neither oseltamivir nor oseltamivir carboxylate is a substrate for, or inhibitor of, cytochrome p450 isoforms. Biotransformation: Hepatic; oseltamivir, ethyl ester prodrug, undergoes extensive hydrolysis to the active aster form, oseltamivir carboxylate. Biological Half-Life Plasma concentrations of oseltamivir declined with a half-life of 1 to 3 hours in most subjects after oral administration, although plasma concentrations of oseltamivir carboxylate declined with a half-life of 6 to 10 hours in most subjects after oral administration. Elimination: 1 to 3 hours for oseltamivir and 6 to 10 hours for oseltamivir carboxylate. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In clinical trials of oseltamivir, serum aminotransferase elevations occurred in 2% of treated subjects, but were asymptomatic and transient in all and there were no reports of clinically apparent liver injury with jaundice. The rates of ALT elevations with oseltamivir were generally similar to those treated with placebo or with a comparative agents. Since its approval in 1999, oseltamivir has been widely used during influenza seasonal outbreaks. There have been a few, isolated reports of mild liver injury in patients receiving oseltamivir, but the relationship of the injury with oseltamivir has not always been very convincingly shown. There have been no reports of acute liver failure or chronic liver disease attributed to oseltamivir use. Furthermore, a proportion of patients with influenza have serum enzyme elevations and even mild jaundice during the acute illness, independent of any therapy. Likelihood score: D (possible rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited data indicate that oseltamivir and its active metabolite are poorly excreted into breastmilk. Maternal dosages of 150 mg daily produce low levels in milk and would not be expected to cause any adverse effects in breastfed infants. Infants over 2 weeks of age can receive oseltamivir directly in doses much larger than those in breastmilk. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding The binding of the active oseltamivir carboxylate metabolite to human plasma protein is negligible at approximately 3 % while the binding of oseltamivir to human plasma protein is 42%, which is insufficient to cause significant displacement-based drug interactions. Interactions Concomitant administration /with probenecid/ results in an approximate two-fold increase in the active metabolite due to a decrease in active anionic tubular secretion in the kidney. In vitro studies demonstrate that neither oseltamivir nor oseltamivir carboxylate is a good substrate for P450 mixed-function oxidases or for glucuronyl transferases. Cimetidine, a non-specific inhibitor of cytochrome P450 isoforms and competitor for renal tubular secretion of basic or cationic drugs, has no effect on plasma levels of oseltamivir or oseltamivir carboxylate. Coadministration with amoxicillin does not alter plasma levels of either compound, indicating that competition for the anionic secretion pathway is weak. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Antiviral Agents; Enzyme Inhibitors At this time, CDC recommends the use of oseltamivir or zanamivir for the treatment of infection with swine influenza (H1N1) viruses. MEDICATION: Antiviral; Orally active inhibitor of influenza virus neuraminidase; converted in vivo to the active acid metabolite, GS-4071. Oseltamivir is indicated for the treatment of uncomplicated acute infection caused by influenza A virus in patients older than 1 year of age who have been symptomatic for no more than 2 days. /Included in US product labeling/ For more Therapeutic Uses (Complete) data for OSELTAMIVIR (8 total), please visit the HSDB record page. Drug Warnings Swine influenza (H1N1) viruses contain a unique combination of gene segments that have not been reported previously among swine or human influenza viruses in the US or elsewhere. The H1N1 viruses are resistant to amantadine and rimantadine but not to oseltamivir or zanamivir. Adverse effects occurring in 1% or more of adults and at an incidence greater than that with placebo include nausea, vomiting, bronchitis, insomnia, and vertigo. Nausea, with or without vomiting, was most common, usually occurring after the initial dose and resolving within 1-2 days, but resulting in drug discontinuance in less than 1% of adults. Adverse effects occurring in 1% or more of children and at an incidence greater than with placebo include vomiting, abdominal pain, epistaxis, otic disorder, and conjunctivitis. Unlike amantadine and rimantadine, neuraminidase inhibitors like oseltamivir do not appear to adversely affect the CNS. FDA Pregnancy Risk Category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./ Serious bacterial infections may begin with influenza-like symptoms or may coexist with or occur as complications during the course of influenza. /Oseltamivir/ has not been shown to prevent such complications. For more Drug Warnings (Complete) data for OSELTAMIVIR (7 total), please visit the HSDB record page. Pharmacodynamics There have been postmarketing reports of delirium and abnormal behavior leading to injury, and in some cases resulting in fatal outcomes, in patients with influenza who were receiving oseltamivir. Because these events were reported voluntarily during clinical practice, estimates of frequency cannot be made but they appear to be uncommon based on oseltamivir. These events were reported primarily among pediatric patients and often had an abrupt onset and rapid resolution. The contribution of oseltamivir to these events has not been established. Influenza can be associated with a variety of neurologic and behavioral symptoms that can include events such as hallucinations, delirium, and abnormal behavior, in some cases resulting in fatal outcomes. These events may occur in the setting of encephalitis or encephalopathy but can occur without obvious severe disease. |
| 分子式 |
C16H28N2O4
|
|---|---|
| 分子量 |
312.40
|
| 精确质量 |
312.204
|
| 元素分析 |
C, 61.51; H, 9.03; N, 8.97; O, 20.48
|
| CAS号 |
196618-13-0
|
| 相关CAS号 |
Oseltamivir-d3;1093851-61-6;Oseltamivir acid;187227-45-8; 204255-11-8 (phosphate); Oseltamivir-d3-1;Oseltamivir-d5;1093851-63-8;Oseltamivir-d3 hydrochloride; 196618-13-0; 204255-09-4 (HCl)
|
| PubChem CID |
65028
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
445.4±55.0 °C at 760 mmHg
|
| 熔点 |
109 °C
|
| 闪点 |
223.2±31.5 °C
|
| 蒸汽压 |
0.0±2.4 mmHg at 25°C
|
| 折射率 |
1.529
|
| LogP |
2.52
|
| tPSA |
90.65
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
22
|
| 分子复杂度/Complexity |
418
|
| 定义原子立体中心数目 |
3
|
| SMILES |
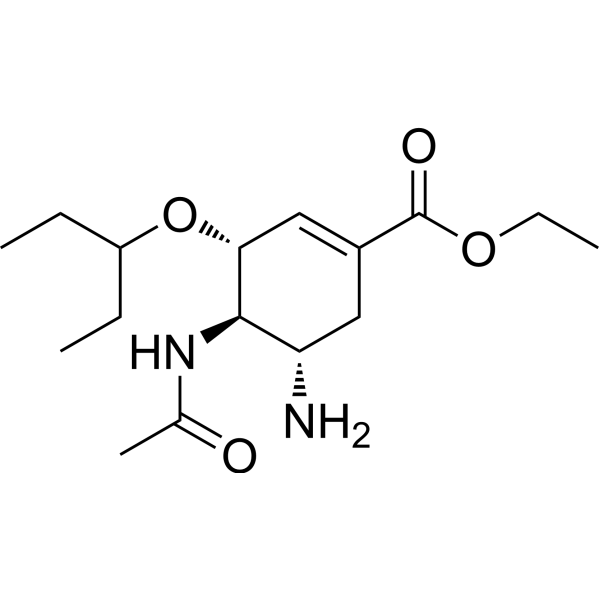
CCC(CC)O[C@@H]1C=C(C[C@@H]([C@H]1NC(=O)C)N)C(=O)OCC
|
| InChi Key |
VSZGPKBBMSAYNT-RRFJBIMHSA-N
|
| InChi Code |
InChI=1S/C16H28N2O4/c1-5-12(6-2)22-14-9-11(16(20)21-7-3)8-13(17)15(14)18-10(4)19/h9,12-15H,5-8,17H2,1-4H3,(H,18,19)/t13-,14+,15+/m0/s1
|
| 化学名 |
ethyl (3R,4R,5S)-4-acetamido-5-amino-3-pentan-3-yloxycyclohexene-1-carboxylate
|
| 别名 |
Tamvir; oseltamivir; Tamiflu-Free; (-)-oseltamivir; GS-4104; GS 4104; oseltamivirum;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 100 mg/mL (320.10 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.00 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.00 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (8.00 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.2010 mL | 16.0051 mL | 32.0102 mL | |
| 5 mM | 0.6402 mL | 3.2010 mL | 6.4020 mL | |
| 10 mM | 0.3201 mL | 1.6005 mL | 3.2010 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。