| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g | |||
| Other Sizes |

| 靶点 |
Neuraminidase; influenza A/H3N2, A/H1N2, A/H1N1, and B viruses
|
|---|---|
| 体外研究 (In Vitro) |
口服治疗后,磷酸奥司他韦(OP)是一种前药,很容易从胃肠道吸收。肝酯酶主要负责前药大量转化为羧酸奥司他韦 (OC) [1]。一种常用的抗流感唾液酸酶抑制剂是磷酸奥司他韦。根据单向方差分析测试,CMA07 和 CMT-U27 细胞系的代谢活性在 305 μM 磷酸奥司他韦处理后显着降低(分别为 p=0.005 和 p<0.0001)。另一方面,与对照细胞相比,当磷酸奥司他韦以 0.305 μM (p=0.9781)、3.05 μM (p=0.7436) 和 30.5 μM (p=0.9623) 处理时,没有观察到统计学上显着的变化。为了评估磷酸奥司他韦对 CMA07 和 CMT-U27 程序性细胞死亡的影响,采用 TUNEL 测试,考虑到 305 μM 磷酸奥司他韦给药会降低细胞代谢活性。与较低奥司他韦浓度或 PBS 相比,24 小时磷酸奥司他韦给药(特别是 305 μM)显着增加了 CMA07 (p=0.001) 和 CMT-U27 (p=0.0002) DNA 断裂[2]。
|
| 体内研究 (In Vivo) |
Ki-67抗原和caspase-3蛋白分别用于评估CMT-U27异种移植肿瘤细胞增殖和凋亡。奥司他韦治疗和未治疗小鼠之间的 Ki-67 和 caspase 3 (p=0.2) 表达几乎没有发现差异 [2]。磷酸奥司他韦治疗的小鼠在原发性肿瘤中显示出明显更多的炎症浸润(p=0.01)。
|
| 酶活实验 |
唾液酸酶活性测定[2]
与良性细胞相比,恶性细胞中唾液酰化水平较高。这可能取决于唾液酸转移酶和唾液酸酯酶的活性。为了评估磷酸奥司他韦对唾液酸酶活性的影响,我们在CMA07和CMT-U27犬乳腺肿瘤细胞中使用一种改良唾液酸(4-methyl-umbelliferyl-Nacetylneuraminic acid-4- munana)进行了体外实验。唾液酸酶的活性是通过获得唾液酸类似物4-MuNana在不同剂量的磷酸奥司他韦处理后转化为荧光化合物甲基-伞形酮(蓝色)来测定的。细胞在12 mm圆形玻璃中培养至融合后,用不同浓度奥司他韦磷酸盐(0.305 μM、3.05 μM、30.5 μM和305 μM奥司他韦磷酸盐溶解PBS)培养液孵育24 h,以药物载体(PBS)为对照。处理24小时后,将每个12mm圆形玻璃放在载玻片上,在2μ m 4-MuNana(2'-(4-甲基伞叶基)-α- d - n -乙酰神经氨酸)溶液中孵育。如前所述,在紫外光(激发波长为360 nm,发射波长为440 nm)下立即用荧光显微镜观察载玻片。用卡尔蔡司荧光显微镜分析载玻片并拍摄图像。 抗病毒活性[3] 将感染细胞(0.01 MOI)培养在含有增加利巴韦林或磷酸奥司他韦浓度的Opti-MEM (2 μg/mL TPCK-trypsin)中。在感染后约24 h (p.i.),去除等分,测定高斯荧光素酶活性。 |
| 细胞实验 |
细胞形态分析[2]
CMA07和CMT-U27细胞以每孔1x104个细胞的密度在6孔板中进行三次镀。以PBS为对照,研究了0.305 μM、3.05 μM和30.5 μM的磷酸奥司他韦浓度。在7天的时间里,使用对比倒置显微镜进行细胞融合和形态学分析。照片是在第0天和第7天在200倍放大镜下拍摄的。 细胞增殖试验[2] 以0.305 μM、3.05 μM、30.5 μM和305 μM磷酸奥司他韦和PBS为对照,分别在24孔板上培养CMA07和CMT-U27细胞。连续7天,每天在Neubauer氏室中以0.4%台锥蓝1:2的比例对细胞进行计数,细胞计数使用体积转换因子为1mm3,即1x104。实验重复3次,记录生长曲线。 细胞生长试验[2] 在CMA07和CMT-U27细胞系中,使用市售的CellTiter 96水溶液试剂测定细胞生长,并根据制造商的说明进行。简单地说,细胞以每孔5x103个细胞的密度在96孔板中一式三次镀。细胞贴壁后,分别以0.305 μM、3.05 μM、30.5 μM和305 μM的终浓度加入磷酸奥司他韦,以PBS为对照。加入MTS四氮唑试剂测定细胞代谢,在490nm处记录吸光度。在0、2、4、6、8、10、12、24和48小时进行测量。在没有细胞的培养孔中,在时间点0h进行额外的对照测量。实验进行了两次。 TUNEL分析[2] 将CMA07和CMT-U27细胞株培养于6孔板中,分别用不同浓度的磷酸奥司他韦(0.305 μM、3.05 μM、30.5 μM和305 μM, PBS为对照)处理。处理24小时后,收集培养基和tripsinized细胞,2000 rpm离心10分钟。细胞在PBS中洗涤,在冷甲醇中固定20分钟。固定后,将细胞重新悬浮于1ml PBS中进行细胞自旋处理。简单地说,使用聚赖氨酸包被载玻片将100 μL细胞悬液在cytospin3离心机中离心。然后根据制造商的说明,使用市售试剂盒(原位细胞死亡检测试剂盒,罗氏荧光素)标记DNA双链断裂,将载玻片用于原位细胞死亡检测。在488nm激发波长的荧光显微镜下观察载玻片,用ImageJ软件记录TUNEL阳性细胞与总细胞的比值,计算死亡细胞的百分比。本实验进行了两次。 愈合试验 在延时显微镜下,使用良性(CMA07)和高度转移(CMT-U27)犬乳腺肿瘤细胞系进行伤口愈合试验。简单地说,将20x104个细胞镀在24孔培养板上,达到高汇合后,用移液管尖端制作人工“伤口”。用磷酸奥司他韦浓度分别为0.305 μM、3.05 μM和30.5 μM的奥司他韦替换培养基,PBS作为对照。使用Axio Vision Release 4.8.2程序,每隔5分钟采集伤口图像,持续48小时。然后转换成视频。305 μM的奥司他韦磷酸盐由于其先前显示的细胞毒性而未进行细胞处理。本实验进行了两次。 荧光细胞化学[2] 细胞在玻璃罩中培养,培养基中分别添加0.305 μM、3.05 μM和30.5 μM的磷酸奥司他韦和PBS作为对照,培养24小时。然后用PBS洗涤细胞,用冷甲醇固定20分钟。固定后,用PBS重新水化细胞,并用10% BSA封闭20分钟。将植物凝集素SNA、MAL I和MAL II(生物素化的amurensis凝集素II, B-1265, Vector Laboratories)在5% BSA的PBS中稀释1:300,在载玻片上室温孵育1小时。然后用PBS洗涤三次,用链亲和素- fitc孵育1小时。PBS洗涤2次后,载玻片与DAPI在PBS中孵育10分钟,载玻片贴载于Vectashield贴载介质中进行荧光分析。在卡尔蔡司荧光显微镜下对载玻片进行分析并拍摄图像。 Western Blot分析[2] 将CMA07和CMT-U27细胞系细胞培养在6个孔板中汇合,在0.305 μM、3.05 μM和30.5 μM磷酸奥司他韦培养基中加入不同浓度的磷酸奥司他韦。孵育24小时后,用PBS洗涤细胞3次,用RIPA裂解缓冲液(50 mM Tris HCl, pH 8;150mm NaCl;NP-40 1%;0.5%去氧乙酸钠;0.1% SDS)含有完整的蛋白酶抑制剂混合物,1mM PMSF(苯基甲基磺酰氟)和1mM Na3VO4(正钒酸钠)。根据制造商的说明,使用Pierce BCA蛋白测定试剂盒的生物辛酸法测定蛋白质浓度。 |
| 动物实验 |
Experimental mice groups and drug treatment[2]
Female NIH(S)II-nu/nu nude mice, aged 4–6 weeks, were orthotopically inoculated with 1 x 106 viable CMT-U27 canine breast cancer cells in the mammary fat pad using a 25 gauge needle. A total of 8 mice were inoculated. When nodules reached a volume of approximately 500mm3, mice (n = 8) were randomized and divided into control group (n = 4) and treatment group (n = 4).The animals received intraperitoneally (IP) dailly either 100 μL of PBS (control group) or 100mg/Kg of Oseltamivir phosphate purchased from the pharmacy, diluted in PBS (treatment group) until time of death. Tumor size was measured using calipers, and tumor volume (mm3) was estimated by width x length x height. To observe metastization, primary tumors of all mice were surgically removed when a mean volume of ~1000–1500mm3 was reached. Mice were anesthetized by IP administration of 100 μL of a mixture containing 50 mg/kg of Ketamin (IMALGENE 1000) and 1 mg/kg of medetomidine hydrochloride (Medetor) and the tumor was excised. We used 2.5 mg/kg of atipamezole (Revertor) per mice to antagonize the effect of anesthesia. Mice were treated with an oral solution of 10 mg/kg of tramadol chloridrate (Tramal) every 8h for 24–48h to prevent pain. Animals were followed up after surgical excision of primary tumors for invasion and/or metastization signs. Mouse Infections[3] Female BALB/c mice (4 to 6 weeks old) were inoculated intranasally with the indicated amount of virus in 30 μL PBS under light isoflurane anesthesia. Body weight was monitored daily. Mice losing 20% of their original body weight were humanely euthanized. At the indicated time, the mice were euthanized, and the lungs were removed for further analysis. Viral load in lung homogenates was determined by both TCID50 and the luciferase assay. For antiviral treatments, mice were treated with either 80 mg/kg/day of ribavirin or 20–50 mg/kg/day of oseltamivir phosphate in PBS, administered by intraperitoneal injection. The treatments were started 2 h before infection and were given twice daily until the end of the experiment. |
| 药代性质 (ADME/PK) |
Absorption
Oseltamivir is readily absorbed from the gastrointestinal tract after oral administration of oseltamivir phosphate and is extensively converted by predominantly hepatic esterases to the active metabolite oseltamivir carboxylate. At least 75 % of an oral dose reaches the systemic circulation as the active metabolite. Exposure to the pro-drug is less than 5 % relative to the active metabolite. Plasma concentrations of both pro-drug and active metabolite are proportional to dose and are unaffected by co-administration with food. Pharmacokinetic parameters following twice daily dosing of oseltamivir 75mg capsules are as follows: Cmax of oseltamivir and oseltamivir carboxylate were found to be 65ng/mL and 348ng/mL, respectively, while AUC (0-12h) of oseltamivir and oseltamivir carboxylate were found to be 112ng·h/mL and 2719ng·h/mL, respectively. Route of Elimination Following absorption, oseltamivir is more than 90 % eliminated through conversion to oseltamivir carboxylate and subsequent elimination entirely through renal excretion. During clinical studies, less than 20 % of oral radiolabelled dose was found to be eliminated in faeces. Volume of Distribution The mean volume of distribution at steady state of the oseltamivir carboxylate ranges approximately between 23 and 26 liters in humans, a volume that is roughly equivalent to extracellular body fluid. Since neuraminidase activity is extracellular, oseltamivir carboxylate distributes to all sites of influenza virus spread. Clearance Renal clearance (18.8 l/h) of the drug exceeds glomerular filtration rate (7.5 l/h) indicating that tubular secretion occurs in addition to glomerular filtration. Protein binding: Oseltamivir phosphate: Moderate (42%). Oseltamivir carboxylate: Very low < 3%. Oseltamivir carboxylate: Volume of distribution is 23 to 26 liters following intravenous administration in 24 subjects. Oral oseltamivir phosphate is readily absorbed then extensively converted to oseltamivir carboxylate, the active form, predominantly by hepatic esterases. At least 75% of an oral dose reaches the systemic circulation as oseltamivir carboxylate. Less than 5% of an oral dose reaches the systemic circulation as oseltamivir phosphate. Elimination: Renal: Oseltamivir carboxylate is extensively eliminated by renal excretion (> 99%). Renal clearance (18.8 L/hr) exceeds glomerular filtration rate (7.5 L/hr), indicating that tubular secretion occurs. Fecal: Elimination of an oral radiolabeled dose in < 20% in the feces. For more Absorption, Distribution and Excretion (Complete) data for OSELTAMIVIR (8 total), please visit the HSDB record page. Metabolism / Metabolites Oseltamivir is extensively converted to the active metabolite, oseltamivir carboxylate, by esterases located predominantly in the liver. Oseltamivir carboxylate is not further metabolized. Neither oseltamivir nor oseltamivir carboxylate is a substrate for, or inhibitor of, cytochrome P450 isoforms. No phase 2 conjugates of either compound have been identified in vivo. Oseltamivir is extensively converted to oseltamivir carboxylate by esterases located predominantly in the liver. Neither oseltamivir nor oseltamivir carboxylate is a substrate for, or inhibitor of, cytochrome p450 isoforms. Biotransformation: Hepatic; oseltamivir, ethyl ester prodrug, undergoes extensive hydrolysis to the active aster form, oseltamivir carboxylate. Biological Half-Life Plasma concentrations of oseltamivir declined with a half-life of 1 to 3 hours in most subjects after oral administration, although plasma concentrations of oseltamivir carboxylate declined with a half-life of 6 to 10 hours in most subjects after oral administration. Elimination: 1 to 3 hours for oseltamivir and 6 to 10 hours for oseltamivir carboxylate. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In clinical trials of oseltamivir, serum aminotransferase elevations occurred in 2% of treated subjects, but were asymptomatic and transient in all and there were no reports of clinically apparent liver injury with jaundice. The rates of ALT elevations with oseltamivir were generally similar to those treated with placebo or with a comparative agents. Since its approval in 1999, oseltamivir has been widely used during influenza seasonal outbreaks. There have been a few, isolated reports of mild liver injury in patients receiving oseltamivir, but the relationship of the injury with oseltamivir has not always been very convincingly shown. There have been no reports of acute liver failure or chronic liver disease attributed to oseltamivir use. Furthermore, a proportion of patients with influenza have serum enzyme elevations and even mild jaundice during the acute illness, independent of any therapy.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation
Limited data indicate that oseltamivir and its active metabolite are poorly excreted into breastmilk. Maternal dosages of 150 mg daily produce low levels in milk and would not be expected to cause any adverse effects in breastfed infants. Infants over 2 weeks of age can receive oseltamivir directly in doses much larger than those in breastmilk.
◉ Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Drugs and Lactation Database (LactMed)
11.1.5 Interactions
Concomitant administration /with probenecid/ results in an approximate two-fold increase in the active metabolite due to a decrease in active anionic tubular secretion in the kidney.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2305
Hazardous Substances Data Bank (HSDB)
In vitro studies demonstrate that neither oseltamivir nor oseltamivir carboxylate is a good substrate for P450 mixed-function oxidases or for glucuronyl transferases. Cimetidine, a non-specific inhibitor of cytochrome P450 isoforms and competitor for renal tubular secretion of basic or cationic drugs, has no effect on plasma levels of oseltamivir or oseltamivir carboxylate.
Physicians Desk Reference 60th ed, Thomson PDR, Montvale, NJ 2006., p. 2811
Hazardous Substances Data Bank (HSDB)
Coadministration with amoxicillin does not alter plasma levels of either compound, indicating that competition for the anionic secretion pathway is weak.
Protein Binding
The binding of the active oseltamivir carboxylate metabolite to human plasma protein is negligible at approximately 3 % while the binding of oseltamivir to human plasma protein is 42%, which is insufficient to cause significant displacement-based drug interactions.
|
| 参考文献 |
|
| 其他信息 |
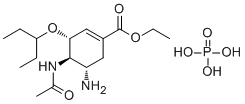
Oseltamivir phosphate is a phosphate salt. It contains an oseltamivir.
Oseltamivir Phosphate is the phosphate salt of oseltamivir, a synthetic derivative prodrug of ethyl ester with antiviral activity. By blocking neuraminidases on the surfaces of influenza viruses, oseltamivir interferes with host cell release of complete viral particles. An acetamido cyclohexene that is a structural homolog of SIALIC ACID and inhibits NEURAMINIDASE. See also: Oseltamivir Acid (has active moiety); Oseltamivir (has active moiety); Oseltamivir carboxylate (has active moiety). Drug Indication Treatment and prevention of influenza |
| 分子式 |
C16H31N2O8P
|
|---|---|
| 分子量 |
410.3997
|
| 精确质量 |
410.181
|
| 元素分析 |
C, 46.83; H, 7.61; N, 6.83; O, 31.19; P, 7.55
|
| CAS号 |
204255-11-8
|
| 相关CAS号 |
Oseltamivir;196618-13-0;Oseltamivir acid;187227-45-8;Oseltamivir-d5 phosphate;Oseltamivir-d3 phosphate
|
| PubChem CID |
78000
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.08g/cm3
|
| 沸点 |
473.3ºC at 760 mmHg
|
| 熔点 |
196-198°C
|
| 闪点 |
240ºC
|
| LogP |
1.448
|
| tPSA |
178.22
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
468
|
| 定义原子立体中心数目 |
3
|
| SMILES |
CCC(CC)O[C@@H]1C=C(C[C@@H]([C@H]1NC(=O)C)N)C(=O)OCC.OP(=O)(O)O
|
| InChi Key |
PGZUMBJQJWIWGJ-ONAKXNSWSA-N
|
| InChi Code |
InChI=1S/C16H28N2O4.H3O4P/c1-5-12(6-2)22-14-9-11(16(20)21-7-3)8-13(17)15(14)18-10(4)19;1-5(2,3)4/h9,12-15H,5-8,17H2,1-4H3,(H,18,19);(H3,1,2,3,4)/t13-,14+,15+;/m0./s1
|
| 化学名 |
ethyl (3R,4R,5S)-4-acetamido-5-amino-3-pentan-3-yloxycyclohexene-1-carboxylate;phosphoric acid
|
| 别名 |
GS-4071, GS-4104, GS4071, GS4104, GS 4071, Oseltamivir phosphate; 204255-11-8; Tamiflu; Oseltamivir (phosphate); Oseltamir Phosphate; Ro 64-0796/002; Oseltamivir (as phosphate); 4A3O49NGEZ; GS 4104, Tamiflu
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~100 mg/mL (~243.66 mM)
DMSO : ~100 mg/mL (~243.66 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.09 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.09 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4366 mL | 12.1832 mL | 24.3665 mL | |
| 5 mM | 0.4873 mL | 2.4366 mL | 4.8733 mL | |
| 10 mM | 0.2437 mL | 1.2183 mL | 2.4366 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。