| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg | |||
| 250mg | |||
| Other Sizes |
| 靶点 |
Bcl-xL (Ki = 0.01 nM); Bcl-2 (Ki = 80 nM)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
A-1155463 会干扰细胞中的 BCL-XL-BIM 复合物,但不会干扰 BCL-2-BIM 复合物。 A-1155463 对 BCL-2 依赖性 RS4;11 细胞没有可检测到的细胞毒性 (EC50>5 mM),但它会杀死 BCL-XL 依赖性 Molt-4 细胞 (EC50=70 nM)。在 BCL-XL 依赖性 H146 细胞中,A-1155463 会导致细胞凋亡的典型迹象,如线粒体释放细胞色素 c、激活 caspase 以及 caspase 依赖性亚 G0-G1 DNA 含量积累所示。
|
||
| 体内研究 (In Vivo) |
A-1155463 多次给药后可抑制体内 H146 小细胞肺癌异种移植肿瘤的生长,并导致小鼠出现基于机制的可逆性血小板减少症。单次腹腔注射给药后,SCID-Beige 小鼠的血小板迅速且可逆地减少,这证明了 A-1155463 能够发挥体内靶向活性。此外,A-1155463 治疗对携带肿瘤的 SCID-Beige 小鼠提供了适度但具有统计显着性的肿瘤生长抑制作用。随附的手稿将对 A-1155463 的活性与不同肿瘤类型的适当化疗相结合进行彻底的机制分析。在非肿瘤 SCID-Beige 小鼠中,单次腹膜内注射 5 mg/kg A-1155463 后,血小板计数显着下降,然后在 72 小时内恢复到正常水平。 SCID-Beige 小鼠接种 BCL-XL 依赖性 H146 肿瘤细胞后,每日腹膜内注射 5 mg/kg 剂量,持续 14 天,可显着抑制肿瘤生长(最大肿瘤生长抑制 = 44%),即停药后缓解。
|
||
| 酶活实验 |
和选择性 BCL-XL 抑制剂;它以皮摩尔亲和力 (Ki 0.01 nM) 与 BCL-XL 结合,同时与 BCL-2 (Ki = 80 nM) 和相关蛋白 BCL-W (Ki = 19 nM) 和 MCL-1 (Ki > 440 nM) 结合>弱1000倍。
使用帝肯Gemini机器人从500μM开始在DMSO中连续稀释化合物。使用Tecan Temo在测定缓冲液中进行1:10的中间稀释,并将10微升转移到白色384孔低容量Corning#3673测定板(2倍起始浓度;10%DMSO)上。然后,将10l蛋白质/探针/抗体混合物以上表所列的最终浓度添加到每个孔中。然后将样品在室温下孵育1小时。对于每种测定,探针/抗体和蛋白质/探针/抗体分别作为阴性和阳性对照包含在每个测定板上。使用340nm激发滤光片和520nm(f-Bak)和495nm(Tb标记的抗His抗体)发射滤光片在Envision上测量时间分辨荧光。解离常数(Ki)使用王方程确定(王Z。描述两个不同配体与蛋白质分子竞争结合的精确数学表达式。FEBS Lett.199536011-114)[2] |
||
| 细胞实验 |
A-1155463 以逐渐增加的浓度给予细胞。按照制造商的说明,72 小时后使用 CellTiter-Glo 发光细胞活力测定法测试细胞的活力。调整结果以反映未经处理的细胞。借助 GraphPad Prism 程序,可以计算 EC50。
细胞增殖和存活率测定[1] 将癌症乳腺细胞系以每孔5000个细胞接种在96细胞板中,并用9×3剂量基质中的化合物组合处理,其中以三倍步骤(20-0.001μM)稀释的navitoclax、venetoclax和a-1155463,以及50、5.0或0.5 nM的多烯紫杉醇。在评估存活率之前,将细胞孵育72小时。NSCLC细胞系在5×5剂量基质中用化合物组合治疗72小时,并如前所述进行评估[1]。 将卵巢癌症细胞系以每孔10000个细胞接种在96细胞板中,并在9×3剂量的基质中用化合物组合处理48小时。以三倍的步骤稀释多西他赛(10-1.1 nM)。Navitoclax、venetoclax和A-1155463分两步稀释(20-0.08μM)。[1] 菌落形成试验[1] 来源于正常人骨髓(BM)的造血前体细胞与不同浓度的navitoclax、venetoclax或A-1155463加上或减去5nM多烯紫杉醇在MethoCult 4230甲基纤维素基培养基中孵育,所述培养基补充有30ng mL-1重组人粒细胞集落刺激因子(rhGCSF)。DMSO用于制备所有测试化合物的储备溶液,并在所有孔中以<0.002%的终浓度存在。将来自三个不同批次(BM07B21195、BM0080512A和BM5H09)的冷冻BM光密度细胞在37°C下快速解冻,在补充了2%胎牛血清(IMDM+2%FBS)的10 mL Iscove改良Dulbecco's培养基(IMDM)中洗涤一次,然后重新悬浮在IMDM+2%FBS中。将2.4-4.3×104个活细胞接种在6孔板的每个孔中,并在37°C(5%CO2)下在试验化合物的存在下孵育14-16天。由训练有素的技术人员使用光学显微镜计数包含至少30个粒细胞的集落形成单元。每种条件都进行了三次测试,以确定平均菌落数+/-一个标准差。 |
||
| 动物实验 |
|
||
| 参考文献 |
|
||
| 其他信息 |
The BCL-2/BCL-XL/BCL-W inhibitor ABT-263 (navitoclax) has shown promising clinical activity in lymphoid malignancies such as chronic lymphocytic leukemia. However, its efficacy in these settings is limited by thrombocytopenia caused by BCL-XL inhibition. This prompted the generation of the BCL-2-selective inhibitor venetoclax (ABT-199/GDC-0199), which demonstrates robust activity in these cancers but spares platelets. Navitoclax has also been shown to enhance the efficacy of docetaxel in preclinical models of solid tumors, but clinical use of this combination has been limited by neutropenia. We used venetoclax and the BCL-XL-selective inhibitors A-1155463 and A-1331852 to assess the relative contributions of inhibiting BCL-2 or BCL-XL to the efficacy and toxicity of the navitoclax-docetaxel combination. Selective BCL-2 inhibition suppressed granulopoiesis in vitro and in vivo, potentially accounting for the exacerbated neutropenia observed when navitoclax was combined with docetaxel clinically. By contrast, selectively inhibiting BCL-XL did not suppress granulopoiesis but was highly efficacious in combination with docetaxel when tested against a range of solid tumors. Therefore, BCL-XL-selective inhibitors have the potential to enhance the efficacy of docetaxel in solid tumors and avoid the exacerbation of neutropenia observed with navitoclax. These studies demonstrate the translational utility of this toolkit of selective BCL-2 family inhibitors and highlight their potential as improved cancer therapeutics.[1]
A-1155463, a highly potent and selective BCL-XL inhibitor, was discovered through nuclear magnetic resonance (NMR) fragment screening and structure-based design. This compound is substantially more potent against BCL-XL-dependent cell lines relative to our recently reported inhibitor, WEHI-539, while possessing none of its inherent pharmaceutical liabilities. A-1155463 caused a mechanism-based and reversible thrombocytopenia in mice and inhibited H146 small cell lung cancer xenograft tumor growth in vivo following multiple doses. A-1155463 thus represents an excellent tool molecule for studying BCL-XL biology as well as a productive lead structure for further optimization.[2] Defects in programmed cell death, or apoptosis, are a hallmark of cancer. The anti-apoptotic B-cell lymphoma 2 (BCL-2) family proteins, including BCL-2, BCL-X(L), and MCL-1 have been characterized as key survival factors in multiple cancer types. Because cancer types with BCL2 and MCL1 amplification are more prone to inhibition of their respectively encoded proteins, we hypothesized that cancers with a significant frequency of BCL2L1 amplification would have greater dependency on BCL-X(L) for survival. Methods: To identify tumor subtypes that have significant frequency of BCL2L1 amplification, we performed data mining using The Cancer Genome Atlas (TCGA) database. We then assessed the dependency on BCL-X(L) in a panel of cell lines using a selective and potent BCL-X(L) inhibitor, A-1155463, and BCL2L1 siRNA. Mechanistic studies on the role of BCL-X(L) were further undertaken via a variety of genetic manipulations. Results: We identified colorectal cancer as having the highest frequency of BCL2L1 amplification across all tumor types examined. Colorectal cancer cell lines with BCL2L1 copy number >3 were more sensitive to A-1155463. Consistently, cell lines with high expression of BCL-XL and NOXA, a pro-apoptotic protein that antagonizes MCL-1 activity were sensitive to A-1155463. Silencing the expression of BCL-X(L) via siRNA killed the cell lines that were sensitive to A-1155463 while having little effect on lines that were resistant. Furthermore, silencing the expression of MCL-1 in resistant cell lines conferred sensitivity to A-1155463, whereas silencing NOXA abrogated sensitivity. Conclusions: This work demonstrates the utility of characterizing frequent genomic alterations to identify cancer survival genes. In addition, these studies demonstrate the utility of the highly potent and selective compound A-1155463 for investigating the role of BCL-X(L) in mediating the survival of specific tumor types, and indicate that BCL-X(L) inhibition could be an effective treatment for colorectal tumors with high BCL-X(L) and NOXA expression.[3] |
| 分子式 |
C35H32FN5O4S2
|
|
|---|---|---|
| 分子量 |
669.79
|
|
| 精确质量 |
669.187
|
|
| 元素分析 |
C, 62.76; H, 4.82; F, 2.84; N, 10.46; O, 9.55; S, 9.57
|
|
| CAS号 |
1235034-55-5
|
|
| 相关CAS号 |
|
|
| PubChem CID |
59447577
|
|
| 外观&性状 |
Off-white to yellow solid
|
|
| 密度 |
1.5±0.1 g/cm3
|
|
| 折射率 |
1.716
|
|
| LogP |
6.61
|
|
| tPSA |
164Ų
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
11
|
|
| 可旋转键数目(RBC) |
11
|
|
| 重原子数目 |
47
|
|
| 分子复杂度/Complexity |
1150
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
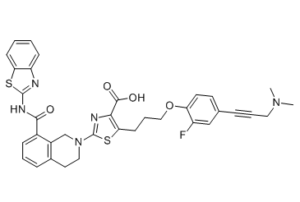
S1C(=C(C(=O)O)N=C1N1CC2C(C(NC3=NC4=CC=CC=C4S3)=O)=CC=CC=2CC1)CCCOC1C=CC(C#CCN(C)C)=CC=1F
|
|
| InChi Key |
SOYCFODXNRVBTI-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C35H32FN5O4S2/c1-40(2)17-6-8-22-14-15-28(26(36)20-22)45-19-7-13-30-31(33(43)44)38-35(47-30)41-18-16-23-9-5-10-24(25(23)21-41)32(42)39-34-37-27-11-3-4-12-29(27)46-34/h3-5,9-12,14-15,20H,7,13,16-19,21H2,1-2H3,(H,43,44)(H,37,39,42)
|
|
| 化学名 |
2-[8-(1,3-benzothiazol-2-ylcarbamoyl)-3,4-dihydro-1H-isoquinolin-2-yl]-5-[3-[4-[3-(dimethylamino)prop-1-ynyl]-2-fluorophenoxy]propyl]-1,3-thiazole-4-carboxylic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (3.73 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (3.73 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (3.73 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4930 mL | 7.4650 mL | 14.9301 mL | |
| 5 mM | 0.2986 mL | 1.4930 mL | 2.9860 mL | |
| 10 mM | 0.1493 mL | 0.7465 mL | 1.4930 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
 (a) Kinetics of platelet reduction and rebound following a single IP dose of A-1155463 in SCID-Beige mice.ACS Med Chem Lett.2014Aug 26;5(10):1088-93. |
|---|
X-ray crystal structure of A-1155463 (green) bound to BCL-XL.ACS Med Chem Lett.2014Aug 26;5(10):1088-93. |
 X-ray crystal structure of compound10(green) bound to BCL-XL.ACS Med Chem Lett.2014Aug 26;5(10):1088-93. |