| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
Endogenous Metabolite; ROS (reactive oxygen species)
|
|---|---|
| 体外研究 (In Vitro) |
在缺乏额外营养支持的情况下,乙酰半胱氨酸通过防止 DNA 断裂来延长胶原蛋白下 PC12 细胞的长期死亡率。乙酰半胱氨酸可保护交感神经元和 PC12 细胞免于死亡 [2]。当暴露于乙酰半胱氨酸时,人主动脉平滑肌细胞会以剂量依赖性方式受损并失去活力[3]。在 PC12 细胞中,乙酰半胱氨酸刺激 Ras 细胞外信号调节器 (ERK)。乙酰半胱氨酸可以防止因缺乏营养而导致的神经元死亡。当乙酰半胱氨酸存在时,一氧化氮 (NO) 更容易从血管组织中的蛋白质结合储备中释放。乙酰半胱氨酸可能会破坏神经突发育和 NGF 依赖性信号传导,表明它可能会破坏氧化敏感的 NGF 机制步骤 [4]。
在本研究中,我们检测了n -乙酰- l-半胱氨酸(LNAC)是否影响营养因子剥夺引起的神经元细胞凋亡。LNAC是一种抗氧化剂,能提高细胞内谷胱甘肽的水平。我们使用了血清缺失的PC12细胞、血清和NGF缺失的PC12细胞和新生儿交感神经元。在每种情况下,LNAC都能防止细胞凋亡DNA断裂,并在缺乏其他营养支持的情况下维持长期存活。与NGF不同,LNAC不会诱导或维持神经突生长或体细胞肥大。为了排除LNAC代谢衍生物的作用,我们评估了n -乙酰- d -半胱氨酸(DNAC)。DNAC还能防止PC12细胞和交感神经元的死亡。然而,其他抗氧化剂在这方面是无效的。由于假设营养因子通过阻止或协调细胞周期进程来预防神经元死亡,我们测试了LNAC或DNAC治疗是否会影响细胞周期。我们发现,这两种抗氧化剂(而不是其他抗氧化剂)抑制PC12细胞的增殖和DNA合成,其浓度与它们防止凋亡的浓度相似。尽管LNAC和DNAC拯救营养因子剥夺引发的细胞凋亡的能力可能源于它们对细胞氧化应激反应的直接影响,但我们的观察结果提出了一种可能涉及细胞周期调节的机制。[2] 吡咯烷二硫代氨基甲酸酯(PDTC)和n -乙酰半胱氨酸(NAC)已被用作抗氧化剂,以防止淋巴细胞、神经元和血管内皮细胞的凋亡。我们在此报道了PDTC和NAC诱导大鼠和人平滑肌细胞凋亡。在大鼠主动脉平滑肌细胞中,PDTC诱导细胞收缩、染色质凝聚和DNA链断裂,与细胞凋亡一致。此外,Bcl-2的过表达抑制了PDTC和NAC引起的血管平滑肌细胞死亡。大鼠主动脉平滑肌细胞活力在PDTC治疗后3小时内下降,12小时时降至30%。PDTC和NAC对平滑肌细胞的影响不是物种特异性的,因为PDTC和NAC都导致大鼠和人主动脉平滑肌细胞活力的剂量依赖性降低。相比之下,PDTC和NAC都没有降低人主动脉内皮细胞的活力。使用抗氧化剂诱导血管平滑肌细胞凋亡可能有助于防止其在动脉硬化病变中的增殖。[3] n -乙酰半胱氨酸(NAC)最近被提议作为人类流行性肺炎的辅助治疗药物。这一建议是基于其在体外限制流感病毒复制和在小鼠模型中减轻疾病严重程度的能力。虽然对不同的病毒(人类和禽类)进行了现有的研究,但与NAC抗流感谱有关的公开信息很少。在这项研究中,我们发现NAC不能改变由接种猪H1N1流感病毒引起的致命性流感肺炎的病程。NAC确实能够在体外抑制猪病毒,但远低于报道的其他菌株。因此,流感病毒对NAC的易感性似乎是菌株依赖的,这表明它不能被认为是流感肺炎的普遍治疗方法。[7] |
| 体内研究 (In Vivo) |
乙酰半胱氨酸(150、300 mg/kg)治疗显着降低了所有治疗组的肝转氨酶,尤其是乙酰半胱氨酸 300 mg/kg 组。乙酰半胱氨酸300 mg/kg组肺谷胱甘肽过氧化物酶显着升高(P=0.04),而其他氧化指标无显着差异[6]。乙酰半胱氨酸可增强 12 个月大的 SAMP8 模型在 T 迷宫避震范式和杠杆估计测试中的认知能力,但不会增强运动产生线索非影响性活动、避免电击的动机或体重 [5]。
氧化应激可能在与年龄相关的神经退行性疾病中起关键作用。在这里,我们检测了两种抗氧化剂,α -硫辛酸(LA)和n -乙酰半胱氨酸(NAC)的能力,以逆转SAMP8小鼠的认知缺陷。到12个月大的时候,这种菌株的β水平升高,学习和记忆能力严重不足。我们发现,与4个月大的小鼠相比,12个月大的SAMP8小鼠的蛋白质羰基(蛋白质氧化指标)水平增加,TBARS(脂质过氧化指标)增加,蛋白质特异性自旋标记MAL-6(氧化诱导的突触体膜蛋白构象变化指标)的弱固定化/强固定化(W/S)比降低。长期服用LA或NAC均可改善12月龄SAMP8小鼠在t迷宫足震避免范式和杠杆按压食欲任务中的认知,而不会对运动活动、避震动机或体重产生非特异性影响。这些影响可能直接发生在大脑内,因为NAC穿过血脑屏障并在大脑中积累。此外,用LA治疗12个月大的SAMP8小鼠可以逆转所有三个氧化应激指标。这些结果支持氧化应激可导致认知功能障碍的假设,并为抗氧化剂的治疗作用提供证据。[5] NAC 300组肝脏组织学评分显著高于对照组(1.7±0.5比2.9±1.1,P = 0.05)。此外,NAC处理显著降低了各处理组的肝脏转氨酶,以NAC 300组居多。NAC治疗组血浆丙二醛水平较低,但无统计学意义。NAC 300组肺谷胱甘肽过氧化物酶显著升高(P = 0.04),而其他氧化生物标志物无显著差异。 结论:NAC对IIR后肝损伤具有显著的保护作用,且可能独立于肠道保护作用。再灌注前额外给予NAC没有进一步的益处。比较方案中最有效的方案是缺血前300 mg/kg。[6] |
| 酶活实验 |
NAC (n -acetyl- l-半胱氨酸)常用来鉴定和检测活性氧诱导剂,并抑制活性氧。在本研究中,我们发现蛋白酶体抑制剂的抑制作用是NAC的一种新活性。NAC和过氧化氢酶(另一种已知的活性氧清除剂)同样抑制活性氧水平和与h2o2相关的细胞凋亡。然而,只有NAC,而不是过氧化氢酶或另一种ROS清除剂Trolox,能够阻止与蛋白酶体抑制相关的作用,如蛋白质稳定、细胞凋亡和泛素偶联物的积累。这些观察结果表明NAC具有ROS抑制剂和蛋白酶体抑制剂的双重活性。最近,NAC被用作一种ROS抑制剂,在功能上表征了一种新的抗癌化合物胡椒明,导致其被描述为ROS诱导剂。相比之下,我们自己的实验表明,该化合物具有蛋白酶体抑制剂的特征,包括抑制FOXM1 (Forkhead box蛋白M1),稳定细胞蛋白,诱导ros非依赖性凋亡和增强泛素偶联物的积累。此外,NAC而非过氧化氢酶或Trolox干扰胡椒隆明的活性,进一步支持胡椒隆明是蛋白酶体抑制剂。最重要的是,我们发现NAC,而不是其他ROS清除剂,直接结合蛋白酶体抑制剂。据我们所知,NAC是已知的第一个直接与蛋白酶体抑制剂相互作用并拮抗活性的化合物。综上所述,本研究的结果表明,由于NAC的双重性质,当NAC被用作抗氧化剂来证明ROS参与药物诱导的细胞凋亡时,数据解释可能并不简单。[1]
我们已经证明n -乙酰半胱氨酸(NAC)在缺乏营养因子的情况下促进交感神经元和嗜铬细胞瘤(PC12)细胞的存活。NAC的这种作用与其抗氧化性能或增加细胞内谷胱甘肽水平的能力无关,而是依赖于正在进行的转录,似乎可归因于NAC作为还原剂的作用。在这里,我们研究了NAC促进神经元存活的机制。我们发现NAC在PC12细胞中激活ras -胞外信号调节激酶(ERK)通路。NAC激活Ras似乎是生存所必需的,因为它不能维持血清缺失的PC12 MM17-26细胞组成性地表达Ras的显性阴性形式。NAC对PC12细胞存活的促进作用被PD98059完全阻断,PD98059是ERK活化MAP激酶/ERK激酶的抑制剂,提示ERK活化在NAC机制中有必要的作用。相比之下,磷脂酰肌醇3-激酶(PI3K)抑制剂LY294002和wortmannin部分阻断ngf促进的PC12细胞存活,对NAC预防死亡没有作用。我们先前假设NAC促进生存的能力与其抗增殖特性相关。然而,尽管NAC不能保护PC12 MM17-26细胞免受营养支持的损失,但它确实抑制了它们合成DNA的能力。因此,NAC的抗增殖作用不需要激活Ras,抑制DNA合成不足以介导NAC促进的生存。这些发现强调了Ras-ERK激活在NAC防止失去营养支持后神经元死亡的机制中的作用。[4] |
| 细胞实验 |
对于存活实验,将洗涤过的细胞重悬于RPM1 1640培养基中,并在涂有大鼠尾胶原的24孔塑料培养皿中以每孔8-10×105的密度接种0.5mL。为了喂养,但为了避免漂浮细胞的损失,在第1、5和10天向培养物中加入新鲜培养基(0.2mL)。对于涉及“预处理”PC12细胞的实验,用NGF在补充有1%热in乙酰半胱氨酸衍生马血清的RPM1 1640培养基中预处理培养物1-2周。然后将细胞洗涤并传代到无血清RPM1 1640培养基中[2]。
|
| 动物实验 |
Rats are randomLy allocated into five groups: sham group (n=5), control group with IIR (n=8) and three groups with IIR who are given Acetylcysteine in different dosages: 150 mg/kg intraperitoneally 5 min before ischemia (n=8, group Acetylcysteine 150), 300 mg/kg i.p 5 min before ischemia (n=7, group Acetylcysteine 300), and 150 mg/kg i.p 5 min before ischemia plus 150 mg/kg 5 min before reperfusion (n=7, group Acetylcysteine 150 + 150). After 4 h of reperfusion, the animals are euthanized by exsanguination from the abdominal aorta [6].
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
An 11 g dose in the form of an effervescent tablet for solution reaches a mean Cmax of 26.5 µg/mL, with a Tmax of 2 hours, and an AUC of 186 µg\*h/mL. An oral dose of radiolabelled acetylcysteine is 13-38% recovered in the urine in the first 24 hours, while 3% is recovered in the feces. The volume of distribution of acetylcysteine is 0.47 L/kg. Acetylcysteine has a mean clearance of 0.11 L/hr/kg. Following oral administration (e.g., when used as an antidote for acetaminophen overdosage), acetylcysteine is absorbed from the GI tract. Oral acetylcysteine is rapidly absorbed, but the bioavailability is low (10-30%) due to significant first-pass metabolism. Intact acetylcysteine has a relatively small volume of distribution (0.5 L/kg). Serum concentrations after intravenous administration of an initial loading dose of 150 mg/kg over 15 minutes are about 500 mg/L. A steady state plasma concentration of 35 mg/L (10-90 mg/L) was reached in about 12 hours following the loading dose with a continuous infusion of 50 mg/kg over 4 hours and 100 mg/kg over the next 16 hours. Metabolism / Metabolites Acetylcysteine can be deacetylated by aminoacylase 1 or other undefined deacetylases before undergoing the normal metabolism of cysteine. Following oral inhalation or intratracheal instillation, most of the administered drug appears to participate in the sulfhydryl-disulfide reaction; the remainder is absorbed from the pulmonary epithelium, deacetylated by the liver to cysteine, and subsequently metabolized. Acetylcysteine undergoes rapid deacetylation in vivo to yield cysteine or oxidation to yield diacetylcystine. Biological Half-Life The mean terminal half life of acetylcysteine in adults is 5.6 hours and in pre-term neonates is 11 hours. Following IV administration of acetylcysteine, mean elimination half lives of 5.6 and 11 hours have been reported in adults and in neonates, respectively. The mean elimination half life was increased by 80% in patients with severe liver damage (i.e., alcoholic cirrhosis (Child-Pugh score of 7-13) or primary and/or secondary biliary cirrhosis (Child-Pugh score of 5-11)). |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Acetylcysteine is a simple modified amino acid and appears to be hepatoprotective. In the many studies of acetylcysteine use with acetaminophen overdose as well as with other conditions such as contrast media nephropathy, pulmonary fibrosis, cystic fibrosis and ulcerative colitis, it has not been associated with serum enzyme elevations during therapy or with episodes of clinically apparent liver injury. Since approval of the oral and intravenous forms of acetylcysteine, there have been no published reports of hepatotoxicity and the product label does not mention liver injury as an adverse event. Indeed, acetylcysteine may be beneficial in treating liver diseases in general, although its current indications are limited to acetaminophen overdose or acetaminophen related acute liver injury. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of acetylcysteine during breastfeeding. To avoid infant exposure, nursing mothers may consider pumping and discarding their milk for 30 hours after administration. Acetylcysteine is very minimally absorbed after inhalation, so breastfeeding can be continued and no special precautions are required. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Acetylcysteine is 66-97% protein bound in serum, usually to albumin. Interactions Guinea pigs were treated with daily drug injections as follows: 1 group received 200 mg kanamycin/kg, sc, 1 group received n-acetylcysteine (300 mg/kg, ip) & the 3rd group received n-acetylcysteine followed by kanamycin 1 hr later. After 7-day recovery, thresholds for detection of the compound action potential were measured. N-acetylcysteine alone had no detectable effect on hearing thresholds. Kanamycin alone produced a moderate (10-20 db) hearing loss below 10 khz & a more severe loss above 10 khz. Animals receiving both n-acetylcysteine & kanamycin had severe hearing losses (40-60 db) at all frequencies between 3 & 30 khz. These data indicate that n-acetylcysteine exerts a strong synergistic effect on kanamycin in producing severe hearing loss & cochlear damage. The major side effect of photodynamic therapy (PDT) using photofrin enhanced skin sensitivity for sunlight which persists for 3-8 weeks after injection. Formation of singlet oxygen and radicals is believed to be involved in the basic mechanism of inducing skin damage. Reducing this side effect would make PDT more widely acceptable particularly for palliative use. Hairless dorsal skin patches of mice injected with 10 mg/kg photofrin ip 24 hr before illumination were used to evaluate the effect of increasing light doses. The light was obtained from a halogen lamp and transmitted via a fiber optic to illuminate a field of 2.5 sq cm. After establishing a dose response relationship for single or fractionated light dose illumination of the skin, drugs known to scavenge radicals, quench singlet oxygen or interfere with histamine release were tested for their protective effect. N-Acetylcysteine, a radical scavenger admin ip (1,000 and 2,000 mg/kg) 1 hr before illumination produced a significant decr in skin damage at light doses > 50 J sq cm (protection factor of 1.3-1.8). When N-acetylcysteine was administered in a dose of 500 mg/kg no protection was observed. Fractionated illumination experiments in combination with multiple injections of N-acetylcysteine (1000 mg/kg) also failed to show any protection. The addition of ranitidine, a histamine blocking agent (25-100 mg/kg) given prior to illumination resulted in a limited protection at higher light doses. From this study /results suggest/ that N-acetylcysteine could be of value in amelioration of the photosensitivity in patients with PDT. The influence of acetylcysteine on cisplatin nephrotoxicity was investigated in female Wistar rats. Admin of 0.6 mg cisplatin/100 mg bw was followed by oliguria and proteinuria, as well as a significant incr of blood urea nitrogen concn. The ip admin of 0.6 mg cisplatin/100 g body wt concomitantly with 100 mg acetylcysteine/100 g body wt sc completely abolished the nephrotoxic effects of cisplatin. However, following this, the platinum concn in the kidney was decr significantly by acetylcysteine treatment. This was caused by a enhanced urinary excretion of platinum. The same effect on cisplatin nephrotoxicity appeared when cisplatin and acetylcysteine were dissolved together in a soln prior to injection. It could be shown that in this soln a ligand exchange reaction of cisplatin by acetylcysteine started immediately, resulting in incr renal excretion and decr platinum concn in the kidney. ... /Results show/ that the protective effect of acetylcysteine on cisplatin nephrotoxicity is based on the formation of a complex unsuitable for tubular resorption. ... ... Studies have shown that the in utero admin of alcohol alters the activity of gamma-glutamyl transpeptidase, the major enzyme involved with the break down of glutathione. The implication is that the in utero admin of alcohol interferes with gamma-glutamyl cycle and ultimately alters glutathione levels. ... The in utero admin of alcohol results in a decr in brain and liver glutathione levels in the developing fetus. ... N-Acetylcysteine ... was given to pregnant mothers throughout gestation in a liquid diet concomitantly with a dose of alcohol which produces a decr in body and brain weights. ... N-Acetylcysteine antagonized the effects of alcohol in the developing fetus. Non-Human Toxicity Values LD50 Dog oral 1 g/kg LD50 Rat oral 3 g/kg LD50 Mouse oral > 3 g/kg LD50 Rat oral > 6 g/kg LD50 Dog ip 700 mg/kg |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Antiviral Agents; Expectorants; Free Radical Scavengers ... 113 patients entered into the study were reported to be pregnant at the time of /acetaminophen/ overdose. Follow up including appropriate laboratory and pregnancy data outcome data, was available in 60 cases. Of these, 19 overdosed during the first trimester, 22 during the second trimester and 19 during the third trimester of pregnancy. Of the 24 patients with acetaminophen levels above the acetaminophen overdose nomogram line, 10 were treated with N-acetylcysteine within 10 hr postingestion; eight delivered normal infants, two had elective abortions. Of ten patients treated with N-acetylcysteine 10-16 hr postingestion, five delivered viable infants, two had elective abortions, and three had spontaneous abortions. Of four women treated with N-acetylcysteine 16-24 hr postingestion, one mother died, and there was one spontaneous abortion, one stillbirth, one elective abortion, and one delivery. ... Acetylcysteine is indicated in the treatment of acetaminophen overdose to protect against hepatotoxicity . /Included in US product labeling/ Acetylcysteine is used in current medical practice in conjunction with chest physiotherapy as mucolytic in patients who have viscid or thickened airway mucus. When administered via direct instillation, it is used to loosen impacted mucus plugs during bronchoscopy. Acetylcysteine can irritate the airways and induce bronchospasm when given by inhalation; therefore, it should be administered simultaneously with or following administration of an inhaled beta-adrenergic bronchodilator. /NOT included in US product labeling/ To evaluate the effectiveness and safety of N-acetylcysteine (NAC) in treating chronic hepatitis B patients, 144 patients with chronic hepatitis B (total bilirubin, TBil>170 mmol/L) from several centers were chosen for a randomized and double blind clinical trial. The patients were divided into a NAC group and a placebo group and all of them were treated with an injection containing the same standardized therapeutic drugs. A daily dose of 8 microgram NAC was added to the injection of the NAC group. The trial lasted 45 days. Hepatic function and other biochemistry parameters were checked at the experimental day 0 and days 15, 30, 45. Each group consisted of 72 patients of similar demology and disease characteristics. During the trial, 28 cases of the 144 patients dropped out. In the NAC group, at day 0 and day 30, the TBil were401.7 vs. 149.2 and 160.1+/-160.6. In the placebo group, the TBil on the corresponding days were 384.1+/-134.0 and 216.3+/-199.9. Its decrease in the NAC group was 62% and 42% in the placebo group. At day 0 and day 45 of treatment, the effective PTa increase rate was 72% in the NAC group and 54% in the placebo group. The total effective rate (TBil + PTa) was 90% in the NAC group and 69% in the placebo group. The parameters of the two groups showed a remarkable difference. The rate of side effects was 14% in the NAC and 5% in the placebo groups. NAC can decrease the level of serum TBil, increase the PTa and reduce the time of hospitalization. NAC showed no serious adverse effects during the period of our treatment. We find that NCA is effective and secure in treating chronic hepatitis B patients. Drug Warnings ... /Acetylcysteine/ should be used during pregnancy only when clearly needed. ... Since it is not known if acetylcysteine is distributed into human milk, the drug should be used with caution in nursing women. Anaphylactoid reactions (i.e., acute hypersensitivity reactions such as rash, hypotension, wheezing, and/or dyspnea) have been reported in patients receiving IV acetylcysteine for the treatment of acetaminophen overdosage; in some cases, the anaphylactoid reactions were serious, including death in a patient with asthma. Rash, urticaria, and pruritus are the most frequently reported adverse reactions in patients receiving IV acetylcysteine. Acute flushing and erythema also have occurred; these reactions generally occur 30-60 minutes after initiating the infusion and resolve despite infusion of the drug. Reactions to acetylcysteine that involve manifestations other than flushing and erythema should be considered anaphylactoid reactions and treated as such. Chest tightness and bronchoconstriction have been reported with acetylcysteine. Clinically overt acetylcysteine-induced bronchospasm occurs rarely and unpredictably, even in patients with asthmatic bronchitis or bronchitis complicating bronchial asthma. Occasionally, patients receiving oral inhalation of acetylcysteine develop increased airway obstruction of varying and unpredictable severity. Patients who have had such reactions to previous therapy with acetylcysteine may not react during subsequent therapy with the drug, and patients who have had inhalation treatments with acetylcysteine without incident may react to subsequent therapy. Nausea, vomiting, and other GI symptoms may occur following oral administration of acetylcysteine in the treatment of acetaminophen overdosage. The drug may also aggravate vomiting associated with acetaminophen overdosage. Administration of dilute acetylcysteine solutions may minimize the tendency of the drug to aggravate vomiting. For more Drug Warnings (Complete) data for N-ACETYLCYSTEINE (15 total), please visit the HSDB record page. Pharmacodynamics Acetylcysteine is indicated for mucolytic therapy and in the management of acetaminophen overdose. It has a short duration of action as it is given every 1-8 hours depending on route of administration, and has a wide therapeutic window. Patients should be counselled regarding diluting oral solutions in cola for taste masking, the risk of hypersensitivity, and the risk of upper gastrointestinal hemorrhage. |
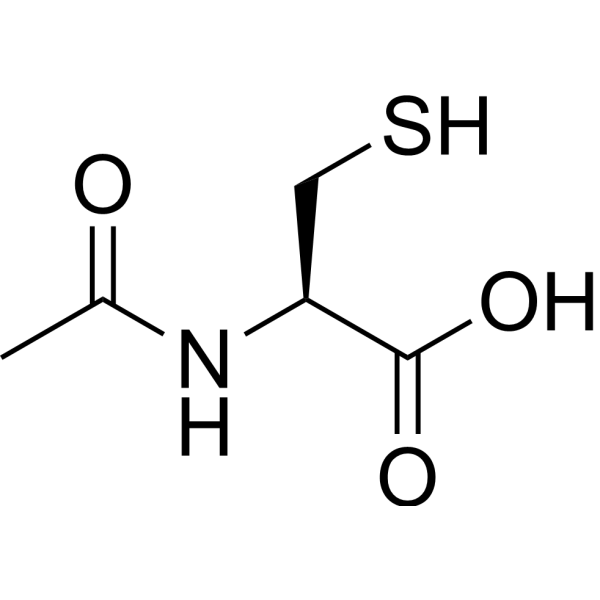
| 分子式 |
C5H9NO3S
|
|---|---|
| 分子量 |
163.1949
|
| 精确质量 |
163.03
|
| 元素分析 |
C, 36.80; H, 5.56; N, 8.58; O, 29.41; S, 19.65
|
| CAS号 |
616-91-1
|
| 相关CAS号 |
Acetylcysteine-d3;131685-11-5;Acetylcysteine-15N
|
| PubChem CID |
12035
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
407.7±40.0 °C at 760 mmHg
|
| 熔点 |
106-108 °C(lit.)
|
| 闪点 |
200.4±27.3 °C
|
| 蒸汽压 |
0.0±2.0 mmHg at 25°C
|
| 折射率 |
1.519
|
| 来源 |
Micro-organism; Ketones, Aldehydes, Acids
|
| LogP |
-0.15
|
| tPSA |
105.2
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
10
|
| 分子复杂度/Complexity |
148
|
| 定义原子立体中心数目 |
1
|
| SMILES |
S([H])C([H])([H])[C@@]([H])(C(=O)O[H])N([H])C(C([H])([H])[H])=O
|
| InChi Key |
PWKSKIMOESPYIA-BYPYZUCNSA-N
|
| InChi Code |
InChI=1S/C5H9NO3S/c1-3(7)6-4(2-10)5(8)9/h4,10H,2H2,1H3,(H,6,7)(H,8,9)/t4-/m0/s1
|
| 化学名 |
Cysteine, N-acetyl-, L-
|
| 别名 |
Acetylcysteine; N-Acetyl-L-cysteine; acetylcysteine; 616-91-1; N-Acetylcysteine; mercapturic acid; Acetadote; L-Acetylcysteine; Broncholysin; Parvolex; Mucosil
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~100 mg/mL (~612.78 mM)
DMSO : ≥ 100 mg/mL (~612.78 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 120 mg/mL (735.34 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
配方 2 中的溶解度: ~120 mg/mL (735 mM) in PBS 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.1278 mL | 30.6391 mL | 61.2783 mL | |
| 5 mM | 1.2256 mL | 6.1278 mL | 12.2557 mL | |
| 10 mM | 0.6128 mL | 3.0639 mL | 6.1278 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Oral N-acetylcysteine for Retinitis Pigmentosa
CTID: NCT05537220
Phase: Phase 3 Status: Recruiting
Date: 2024-11-12
|
|
|