| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
EGFR tyrosine kinase (IC50=0.7 μM).
|
|---|---|
| 体外研究 (In Vitro) |
AG 494 抑制 DHER-14 细胞中 EGF 依赖性 DNA 合成和 Cdk2 激活 [2]。 AG-494 可以显着降低并阻止 H2O2 处理的细胞以及二氧化硅刺激的细胞中 NF-kB 的激活 [4]。 BMP9 产生的 ALP 活性被 AG-494(3-9 μM;5-7 天)以剂量依赖性方式抑制 [5]。
我们之前已经证明,EGFR激酶选择性酪氨酸磷酸酶AG494不能抑制完整细胞中的EGFR激酶。然而,AG494被证明可以抑制EGF或血清诱导的细胞增殖(Osherov等人,J.Biol.Chem.268(1993)11134-11142)。在这篇初步通讯中,我们表明AG 494及其类似物AG 490和AG 555阻断了Cdk2的激活。相比之下,AG 1478是一种更具选择性的EGFR激酶阻断剂,在完整细胞中也具有EGFR激酶阻断活性,但却不能做到这一点。即使在Cdk2活化最大的EGF添加后20小时添加,AG 494也对Cdk2活化具有完全的抑制活性。对Cdk2活化的抑制活性与其DNA合成抑制活性相似,强烈表明其靶点是Cdk2活化涉及的分子机制之一。AG 494及其类似物可能成为开发针对细胞周期机制的药物的有用先导化合物。[2] BMP9诱导的成骨分化可被EGFR抑制剂有效阻断[4] 表皮生长因子通过激活其酪氨酸激酶受体EGFR(又名HER1)启动下游事件。我们试图确定常见的EGFR抑制剂是否会对BMP9诱导的MSCs成骨分化产生任何影响。我们测试了四种EGFR抑制剂,AG494、AG1478、厄洛替尼和吉非替尼,发现它们都以剂量依赖的方式抑制BMP9诱导的ALP活性(图3A)。在四种测试的抑制剂中,厄洛替尼和吉非替尼被临床用作抗癌药物,在抑制BMP9诱导的ALP活性方面,它们被证明比AG494和AG1478略有效(图3A和B)。因此,这些体外研究结果表明,EGFR信号传导可能在BMP9调节的成骨分化中发挥重要作用,尽管这些抑制剂也可能靶向其他酪氨酸激酶。 |
| 酶活实验 |
抑制自磷酸化[2]
在4°C下,将膜(2μg/次测定)与EGF(50 nM)在50 mM HEPES(pH 7.4)、125 mM NaCl中预孵育15分钟。通过向12μl反应混合物中加入8μl膜来启动该测定,该反应混合物含有50 mM HEPES(pH 7.4)、125 mM NaCl、2μM ATP(=2 K M最终)、1μCi[32P]γ-ATP、4 mM MnCl2、24 mM MgCl2和4μl溶于10%Me2SO/45%乙醇/45%DDW(双蒸馏水)的tyrphostin。该测定在4°C下进行,30秒后通过加入8μl沸腾的SDS-PAGE样品缓冲液终止。在6%SDS-PAGE上分离蛋白质,并进行放射自显影。然后可以检测到受体分子量中单个EGF诱导的32P标记带。通过密度测定法对该条带进行扫描和定量。使用E-Z拟合程序计算IC50值。 所有反应均在V形96孔板上使用多吸管快速进行。反应在30秒内均呈线性。控制放射自显影的曝光时间,使胶片曝光呈线性。基础EGF非依赖性活性不超过EGF激活的HER-1/EGF受体自磷酸化的5%。pp60c-src活性的抑制基本上是通过使用DHER-14细胞进行的,如前所述,使用变性烯醇化酶作为底物。如Schechter等人所述,对小麦胚凝集素(WGA)纯化的胰岛素受体的自磷酸化进行抑制。使用由NIH-3T3细胞制备的膜,使用如HER-1所述的用人HER-2/Neu受体转染的已发表程序,对HER-2/Nue受体的自磷酸化进行抑制。 免疫沉淀和Cdk2激酶测定[2] 如所述,DHER-14细胞在DMEM+10%小牛血清的Costar 6孔培养皿中生长融合48小时。细胞被饥饿3天。在最后24小时内,细胞被各种有丝分裂原(20 nM EGF;50 ng/ml TPA佛波酯)或50μM溶血磷脂酸(LPA)激活不同时间。按照每个实验的描述,用tyrphostins孵育细胞。细胞在PBS中洗涤一次,然后在冰上用1 ml/孔冷IP缓冲液(50 mM HEPES、250 mM NaCl、5 mM DTT、0.25%NP-40、10 mM NaF和蛋白酶抑制剂)裂解。将裂解物在冰上孵育30分钟,然后在微量离心机中离心14000×g 15分钟。转移上清液,在饱和浓度为1μg/eppendorf的抗Cdk2抗体存在下孵育2小时。将免疫复合物收集在蛋白A琼脂糖珠上(每eppendorf 7.5μl包装体积),并在IP缓冲液中洗涤3次。然后在激酶缓冲液(50 mM HEPES、10 mM MgCl、1 mM DTT、1μM冷ATP)中第四次洗涤沉淀物。用细孔Hamilton注射器从珠粒中吸出所有缓冲液,每次测定加入40μl/eppendorf反应混合物引发激酶反应,该混合物含有激酶缓冲液和1μCi/测定[γ-32P]ATP和2.5μg组蛋白H1(新鲜制备)。 反应在30°C下进行20分钟,然后通过加入12.5μl/测定SDS-PAGE样品缓冲液×4来停止。样品在10%SDS-PAGE凝胶上分离,并在-70°C下暴露于薄膜约2小时。通过密度测定法进行定量。 |
| 细胞实验 |
抑制[3H]胸苷摄取[2]
将细胞以7000个细胞/孔的速度接种在预涂有1μg/孔人纤维连接蛋白的96孔Costar培养皿中。细胞生长至融合2天。将培养基换成含有0.25%小牛血清的DMEM培养48小时,然后将细胞与20 nM EGF、50μM溶血磷脂酸或50 ng/ml TPA(佛波酯)一起孵育16小时。16小时后,加入[3H]胸苷0.5μCi/ml,持续4小时。在加入有丝分裂原前30分钟或最后4小时,加入不同浓度的溶于10%Me2SO中的Tyrphostin,浓度为×100。通过闪烁计数对三氯乙酸可沉淀物质进行定量。基础EGF非依赖性活性不超过DHER-14细胞丝裂原依赖性[3H]胸苷摄取活性的5%。在添加EGF前30分钟或添加[3H]胸苷时添加Tyrphostins。因此,AG494和AG 1478存在20或4小时。 免疫沉淀和免疫印迹分析[2] 将细胞以2×105个细胞/5 ml/孔的密度铺在Costar 6孔培养皿中,生长至融合2天,然后在含有0.25%小牛血清的2 ml/孔DMEM中饥饿48小时。在饥饿的最后16小时或最后2小时,加入溶于10%Me2SO中×100浓度的Tyrphostin。然后加入EGF(20 nM),在4°C下用EGF(20 nM)孵育平板2分钟,在此期间受体自磷酸化呈线性。通过加入1 ml/孔终止裂解缓冲液终止反应,该缓冲液含有20 mM HEPES(pH 7.4)、125 mM NaCl、1%Triton X-100、5 mM NaF、100μM NaVO3、200μM ZnCl2、1 mM EDTA、2 mM EGTA、1 mM PMSF、10μg/ml抑肽酶、5μg/ml亮肽。如上所述,使用单克隆抗体108进行免疫沉淀。将免疫复合物在沸腾的样品缓冲液中释放,然后在SDS-PAGE 5-15%梯度凝胶上运行样品,转移到硝化纤维中(ABN半干印迹),在TBST(Tris缓冲盐水(pH 7.4),1%吐温-20,5%牛血清白蛋白)中封闭30分钟,然后在TBST中用单克隆抗磷酸酪氨酸抗体PT66在40°C下探测3小时,在TBS中洗涤4次,然后用[125I]山羊抗小鼠抗体(1μCi/ml)重新检测。然后将印迹在TBS中洗涤4次,风干并通过放射自显影进行分析。 |
| 参考文献 | |
| 其他信息 |
Tyrphostin B48 is a member of the tyrphostin family of tyrosine kinase inhibitors that inhibits epidermal growth factor receptor. (NCI)
We have previously shown that the EGFR kinase selective tyrphostin AG494 fails to inhibit EGFR kinase in intact cells. Yet, AG 494 proved to inhibit EGF- or serum-induced cell proliferation (Osherov et al., J. Biol. Chem. 268 (1993) 11134-11142). In this preliminary communication we show that AG 494 as well as its close analogs AG 490 and AG 555 block Cdk2 activation. In contrast, AG 1478, a more selective EGFR kinase blocker which is also active as EGFR kinase blocker in intact cells, fails to do so. AG 494 exerts its full inhibitory activity on Cdk2 activation even when added 20 h subsequent to EGF addition when Cdk2 activation is maximal. The inhibitory activity on Cdk2 activation parallels its DNA synthesis inhibitory activity, strongly suggesting that its target is one of the molecular mechanisms involved in Cdk2 activation. AG 494 and its analogs may become useful lead compounds for the development of drugs aimed at the cell cycle machinery. [2] Mesenchymal stem cells (MSCs) are multipotent progenitors, which give rise to several lineages, including bone, cartilage and fat. Epidermal growth factor (EGF) stimulates cell growth, proliferation and differentiation. EGF acts by binding with high affinity to epidermal growth factor receptor (EGFR) on the cell surface and stimulating the intrinsic protein tyrosine kinase activity of its receptor, which initiates a signal transduction cascade causing a variety of biochemical changes within the cell and regulating cell proliferation and differentiation. We have identified BMP9 as one of the most osteogenic BMPs in MSCs. In this study, we investigate if EGF signalling cross-talks with BMP9 and regulates BMP9-induced osteogenic differentiation. We find that EGF potentiates BMP9-induced early and late osteogenic markers of MSCs in vitro, which can be effectively blunted by EGFR inhibitors Gefitinib and Erlotinib or receptor tyrosine kinase inhibitors AG-1478 and AG494 in a dose- and time-dependent manner. Furthermore, EGF significantly augments BMP9-induced bone formation in the cultured mouse foetal limb explants. In vivo stem cell implantation experiment reveals that exogenous expression of EGF in MSCs can effectively potentiate BMP9-induced ectopic bone formation, yielding larger and more mature bone masses. Interestingly, we find that, while EGF can induce BMP9 expression in MSCs, EGFR expression is directly up-regulated by BMP9 through Smad1/5/8 signalling pathway. Thus, the cross-talk between EGF and BMP9 signalling pathways in MSCs may underline their important roles in regulating osteogenic differentiation. Harnessing the synergy between BMP9 and EGF should be beneficial for enhancing osteogenesis in regenerative medicine. [4] Nuclear factor-kB (NF-kB) is a multiprotein complex that may regulate a variety of inflammatory cytokines involved in the initiation and progression of silicosis. The present study documents the ability of in vitro silica exposure to induce DNA-binding activity of NF-kB in a mouse peritoneal macrophage cell line (RAW264.7 cells) and investigates the role of reactive oxygen species (ROS) and/or protein tyrosine kinase in this activation. In vitro exposure of mouse macrophages to silica (100 µg/ml) resulted in a twofold increase in ROS production, measured as the generation of chemiluminescence (CL), and caused activation of NF-kB. Silica-induced CL was inhibited 100% by superoxide dismutase (SOD) and 75% by catalase, while NF-kB activation was inhibited by a variety of antioxidants (catalase, superoxide dismutase, alpha-tocopherol, pyrrolidine dithiocarbamate, or N-acetylcysteine). Further evidence for the involvement of ROS in NF-kB activation is that 1 mM H2O2 enhanced NF-kB/DNA binding and that this activation was inhibited by catalase. Specific inhibitors of protein tyrosine kinase, such as herbimycin A, genistein, and AG494, prevented NF-kB activation in silica-treated cells. Genistein and AG494 also reduced NF-kB activation in H2O2-treated cells. Results con firm that tyrosine phosphorylation of several cellular proteins (approximate molecular mass of 39, 58?70, and 103 kD) was increased in silica-exposed macrophages and that genistein inhibited this silica-induced phosphorylation. In contrast, inhibitors of protein kinase A or C, such as H89, staurosporin, calphostin C, and H7, had no marked inhibitory effect on silica-induced NF-kB activation. The results suggest that ROS may play a role in silica-induced NF-kB activation in macrophages and that phosphorylation events mediated by tyrosine kinase may be involved in this activation.[5] |
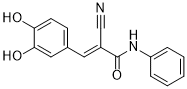
| 分子式 |
C16H12N2O3
|
|---|---|
| 分子量 |
280.2781
|
| 精确质量 |
280.084
|
| 元素分析 |
C, 68.56; H, 4.32; N, 9.99; O, 17.12
|
| CAS号 |
133550-35-3
|
| PubChem CID |
5328771
|
| 外观&性状 |
Yellow to brown solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
586.9±50.0 °C at 760 mmHg
|
| 熔点 |
249 °C(dec.)
|
| 闪点 |
308.8±30.1 °C
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
| 折射率 |
1.736
|
| LogP |
2.4
|
| tPSA |
93.35
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
21
|
| 分子复杂度/Complexity |
446
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O([H])C1=C(C([H])=C([H])C(/C(/[H])=C(\C#N)/C(N([H])C2C([H])=C([H])C([H])=C([H])C=2[H])=O)=C1[H])O[H]
|
| InChi Key |
HKHOVJYOELRGMV-XYOKQWHBSA-N
|
| InChi Code |
InChI=1S/C16H12N2O3/c17-10-12(8-11-6-7-14(19)15(20)9-11)16(21)18-13-4-2-1-3-5-13/h1-9,19-20H,(H,18,21)/b12-8+
|
| 化学名 |
2E-cyano-3-(3,4-dihydroxyphenyl)-N-phenyl-2-propenamide
|
| 别名 |
AG-494; AG 494; 133550-35-3; Tyrphostin B48; alpha-Cyano-(3,4-dihydroxy)-N-phenylcinnamide; (E)-2-cyano-3-(3,4-dihydroxyphenyl)-N-phenylacrylamide; 139087-53-9; AG494; Tyrphostin AG-494.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~356.79 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.92 mM) (饱和度未知) in 10% DMSO + 40% PEG300 +5% Tween-80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80+,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.5679 mL | 17.8393 mL | 35.6786 mL | |
| 5 mM | 0.7136 mL | 3.5679 mL | 7.1357 mL | |
| 10 mM | 0.3568 mL | 1.7839 mL | 3.5679 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。