| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
CBFβ-SMHHC
|
|---|---|
| 体外研究 (In Vitro) |
AI-10-49 的 IC50 值为 0.26 μM,可阻断 CBFβ-SMMHC 与 RUNX1 Runt 结构域的结合 [1]。 AI-10-49(1 μM;3、6、12 小时)以特定方式减少 CBFβ-SMMHC 与 RUNX1 的结合[1]。
肝微粒体稳定性的测量表明,AI-10-47降低了代谢不稳定性,因此证明了二价衍生物AI-10-49的合成是合理的(表1)。AI-10-49是强效的(FRET IC50=260nM)(表1)[等温滴定量热法(ITC)测量得出解离常数(KD)=168 nM](图S6),改善了体内药代动力学特性(t½=380min)(图S5),与母体质子化二价化合物AI-4-83(IC50约为3μM)相比,对ME-1细胞生长的抑制活性增强(IC50=0.6 mM)(图1F)(图1E)。请注意,AI-10-49在正常人骨髓细胞中显示出可忽略的活性(IC50>25μM)(图1G),这表明了一个强大的潜在治疗窗口。在11种人类白血病细胞系中,ME-1细胞是唯一对AI-10-49高度敏感的细胞系(图S7)。 在ME-1细胞中评估了AI-10-49在破坏内源性RUNX1与CBFβ-SMMHC结合方面的特异性。AI-10-49有效地将DRUNX1与CBFβ-SMMHC分离,治疗6小时后分离率为90%,而它对CBFβ-RUNX1的结合只有适度的影响(图2A)。AI-10-49不影响RUNX1、CBFβ和CBFβ-SMMHC的稳定性(图S8A)。在inv(16)AML中,CBFβ-SMMHC抑制了RUNX1调控基因RUNX3、CSF1R和CEBPA的表达。先前的研究表明,在CBFβ-SMMHC存在的情况下,RUNX1与靶基因的结合减少,这表明CBFβSMMHC通过阻断RUNX1与靶标DNA位点的结合来抑制RUNX1靶基因(图2B)。与该模型一致,染色质免疫沉淀(ChIP)检测表明,用AI-10-49处理ME-1细胞6小时,RUNX3、CSF1R和CEBPA启动子处的RUNX1占有率分别增加了8倍、2.2倍和8倍,而在对照位点没有观察到富集(图2C和图S8、B和C)。据此,用AI-10-49处理ME-1细胞6或12小时可增加RUNX3、CSF1R和CEBPA的表达,但对对照基因PIN1没有影响(图2D)。在inv(16)阴性的U937细胞中没有观察到这些效应。这些数据确立了AI-10-49在抑制CBFβ-SMMHC与RUNX1结合方面的选择性,并验证了我们使用二价抑制剂实现这种特异性的方法[1]。 为了测试AI-10-49在人类inv(16)白血病治疗中的潜在效用,我们评估了用单价AI-10-47和二价AI-10-49剂量范围治疗48小时的四种原发inv(6)AML细胞样本的存活率。如图3B所示,用浓度为5和10μM的AI-10-49处理后,inv(16)患者细胞的存活率降低(个体剂量反应实验如图S12所示)。请注意,二价AI-10-49比单价化合物AI-10-47更有效,因此概括了在人类inv(16)细胞系ME-1中观察到的效果。相比之下,正常核型AML样本的存活率不受AI-10-49治疗的影响(图3C)。对另外五组AML样本的分析表明,AI-10-49治疗特异性降低了inv(16)白血病细胞的存活率,但对其分化没有明显影响(图S13)。当我们通过评估化合物暴露后的集落形成单位(CFU)来评估AML细胞形成集落的能力时,AI-10-49的特异性也很明显。与正常核型和t(8;21)AML患者样本相比,AI-10-49选择性降低了inv(16)AML细胞形成CFU的能力(图3D)。这种抑制作用是剂量依赖性的(在5和10μM时分别为40%和60%)(图3E),而用AI-10-47处理的AML细胞、具有正常核型的AML细胞(图3F)或CD34+脐带血细胞(图3G)的CFU没有变化。这些研究表明,AI-10-49选择性抑制inv(16)AML母细胞的存活率和CFU能力,而它对具有正常核型的AML母细胞或重要的是对正常人类造血祖细胞的影响可以忽略不计[1]。 |
| 体内研究 (In Vivo) |
AI-10-49(200 mg/kg;每日)可延缓小鼠白血病的进展[1]。
高达90%的inv AML患者在受体酪氨酸激酶途径的成分中存在协同突变,包括N-RAS和c-Kit。我们最近通过结合条件性NrasLSL-G12D和CbfbMYH11等位基因,开发了一种有效的inv AML小鼠模型。为了在体内测试AI-10-49给药的效果,我们用Cbfb+/MYH11:Ras+/G12D白血病细胞移植小鼠,等待5天植入,然后用载体[二甲亚砜(DMSO)]或200mg/kg体重AI-10-49治疗小鼠10天,并评估其对疾病潜伏期的影响。如图3A所示,载体治疗的小鼠死于白血病的中位潜伏期为33.5天,而AI-10-49治疗的小鼠存活时间明显更长(中位潜伏期=61天;P=0.7×10-6)。因此,用AI-10-49进行短暂治疗可以减少体内白血病的扩大。尽管我们没有评估长期暴露后的毒性,但在服用AI-10-49 7天后,我们没有观察到毒性证据[1]。 |
| 酶活实验 |
FRET检测。[1]
如前所述,Cerulean Runt结构域被表达和纯化。Venus CBFβ-SMMHC是通过将6xHis标签和Venus插入NdeI和NcoI位点之间的pET22b载体中,并在NcoI和BamHI位点之间插入CBFβ-SSMHC(CBFβ/SMMHC构建体包含369个氨基酸,CBFβ为1-166,MYH11为166-369(氨基酸1526-1730))构建的。融合蛋白通过标准镍亲和层析纯化,柱上苯并酶处理以去除残留的DNA污染物。使用前,将蛋白质透析到FRET缓冲液(25mM Tris-HCl,pH 7.5,150mM KCl,2mM MgCl2)中。蛋白质浓度分别通过天蓝色和金星在433和513 nm处的紫外吸光度测定。Cerulean Runt结构域和Venus CBFβ-SMMHC以1:1混合,在96孔黑色COSTAR板中达到10nM的最终浓度。将化合物的DMSO溶液加入至DMSO的最终浓度为5%(v/v),然后在室温下在黑暗中孵育平板一小时。使用PHERAstar微孔板读数器测量荧光(激发波长为433 nm,发射波长为474和525 nm)。对于IC50测定,绘制525nm和474nm处的荧光强度比与化合物浓度的对数,并使用Origin7.0将所得曲线拟合为S形曲线。进行了三次独立的测量,并使用它们的平均值和偏差进行IC50数据拟合。[1] 蛋白质核磁共振波谱[1] 所有核磁共振实验均在30°C下在配备低温探头的布鲁克800 MHz仪器上进行。所有NMR样品均在50 mM磷酸钾、0.1 mM EDTA、0.1 mM NaN3、1 mM DTT和5%(v/v)D2O中制备,最终pH值为7.5。15N1 H HSQC实验使用500µM样品,13C1 H HSQC试验在1 mM样品上进行。所有核磁共振数据均使用NMRPipe和Sparky进行处理。加权化学位移变化(百万分之几)通过以下方程式计算:�HN |+(|∆�N|/4.69)。[1] 等温滴定量热法[1] 将由MYH11(MYH11氨基酸1526-1730)的CBFß残基1-166和166-369组成的369个氨基酸CBFß-SMMHC构建体克隆到具有N端6xHis标签和Tev蛋白酶位点的修饰pET22b载体中,并在30°C下用1mM IPTG诱导的Terrific Broth培养基在Rosetta2(DE3)细胞中表达8小时。CBFß-SMMHC通过Niaffinity色谱纯化,然后进行苯并酶处理和Tev蛋白酶消化过夜。然后将融合蛋白第二次通过Ni-NTA柱,然后进行Q-Sepharose离子交换层析以去除残留的核酸。通过使用Sephacryl S300柱的尺寸排阻色谱实现了额外的纯化。将纯化的CBFß-SMMHC透析到1 L ITC缓冲液(12.5 mM KPi(pH 6.5)、150 mM NaCl、2 mM MgCl2、1 mg/mL NaN3、1 mM DTT和0.25%DMSO)中4小时。将AI-10-49单独加入10 mL ITC缓冲液中,使终浓度达到2µM。使用DSS作为标准,在布鲁克600 MHz NMR光谱仪上使用1H NMR验证了AI-10-49的浓度。所有ITC测量均在30°C下在微量热系统上进行。CBFß-SMMHC和AI-10-49样品脱气20分钟,并在量热池中向400 nM CBFßSMMHC溶液中注射8µL 2µMAI-10-49。ITC数据中一致观察到双相转变,表明有多个过程导致了观察到的热量。因此,由于观察到的热量相对较小,无法使用热量计上可用的标准软件分析这些数据,该软件使用热值来确定KD。相反,使用Origin 7.5对数据进行分析,并在校正稀释焓以得出表观KD值后,将其拟合到单点S形结合曲线。重复测量三次,并将值报告为平均值±标准偏差。 |
| 细胞实验 |
蛋白质印迹分析[1]
细胞类型: ME-1 细胞 测试浓度: 1 μM 孵育时间: > 3、6 小时 实验结果: RUNX1 与 CBFβ-SMMHC 有效解离。 RT-PCR[1] 细胞类型: ME-1 和 U937 细胞 测试浓度: 1 μM 孵育持续时间:6、12 小时 实验结果:RUNX3、CSF1R 和 CEBPA 表达增加。 |
| 动物实验 |
Animal/Disease Models: Mice (Cbfb+/MYH11:Ras+/G12D leukemic cells)[1]
Doses: 200 mg/kg Route of Administration: per day Experimental Results: decreased leukemia expansion in vivo and survived Dramatically long. Leukemia transplantation studies in mice [1] Leukemic cells carrying Cbfb+/MYH11 and Nras+/G12D oncogenic alleles were generated in CD45.2 C57BL/6 mice, as previously described. Briefly, 2x103 Cbfb+/MYH11;Nras+/G12D leukemic cells were transplanted into each of 22 sublethally irradiated six to eight week old CD45.1 C57BL/6 female mice. The number of mice per group was selected in preliminary assays to achieve statistical power under the established experimental conditions. At day five post-transplantation, mice were randomized into two groups, and injected intraperitoneally for ten days with 50 µL DMSO or AI-10-49 (200 mg/kg) in DMSO. Mice were kept under observation by more than one person to determine the median leukemia latency, and were euthanized once signs of disease were detected, including reduced motility and grooming activity, hunched back, pale paws (anemia), and hypothermia. At time of euthanasia, peripheral blood and spleen cell were extracted and analyzed as previously described. Leukemia burden was analyzed in peripheral blood by measuring the total white blood cell counts and the number of cells in the c-kit(+)-gated population. |
| 药代性质 (ADME/PK) |
Analysis of the pharmacokinetic properties of AI-4-57 (analog of AI-10-49) showed that the compound has a short half-life (t½ = 37 min) in mouse plasma (fig. S5) and that loss of the methyl group from the methoxy functionality is the primary metabolite. Trifluoromethoxy (CF3O) substitutions have been shown to be less reactive (18, 19), so we synthesized AI-10-47 with this substitution. FRET measurements show that this substitution actually enhances the activity of the monovalent compound (Table 1). Measurements of stability in liver microsomes showed that AI-10-47 reduced the metabolic liability and so justified the synthesis of the bivalent derivative AI-10-49 (Table 1).AI-10-49 is potent (FRET IC50=260nM) (Table 1) [isothermal titration calorimetry (ITC) measurements yielded a dissociation constant (KD) = 168 nM] (fig. S6), has improved in vivo pharmacokinetic properties (t½ = 380min) (fig. S5), and has enhanced inhibitory activity on ME-1 cell growth (IC50 = 0.6 mM) (Fig. 1F) compared with the parent protonated bivalent compound AI-4-83 (IC50 of ~3 μM) (Fig. 1E). Note that AI-10-49 showed negligible activity (IC50 > 25 μM) in normal human bone marrow cells (Fig. 1G), which indicated a robust potential therapeutic window. In a panel of 11 human leukemia cell lines, ME-1 cells were the only cell line highly sensitive to AI-10-49 (fig. S7). [1]
Pharmacokinetic Studies [1] Prior to the study, mice were fasted at least three hours and water was available ad libitum. Animals were housed on a 12-hour light/dark cycle at 72-74°C and 30-50% relative humidity. For intraperitoneal dosing 24 – 28 gm male C57BL/6 mice were manually restrained and injected in the peritoneal cavity midway between the sternum and pubis and slightly off the midline of the mouse. A 1-cc syringe with a 27-gauge needle was used for each injection. Blood was collected from the animals according to scheduled time points. Animals were anesthetized with isoflurane and blood drawn via cardiac puncture. Blood was immediately transferred to 1.5 mL heparinized microcentrifuge tubes and centrifuged at 4000 rpm for ten minutes. Plasma was then transferred to clean tubes and frozen. Due to exsanguination, the animals did not wake from the anesthesia and death was insured while under anesthesia by thoracotomy. This method is consistent with the recommendations of the AVMA Guidelines on Euthanasia for use of exsanguination as a means of euthanasia. Noncompartmental pharmacokinetic analysis of the test compound plasma concentration-time data was conducted using PK Solutions 2.0. [1] AI-10-49– HPLC analysis was performed with an LC system consisting of a Shimadzu SCL-10Avp controller, SIL-10A autosampler, LC-10ADvp pumps, SPD-10Avp detector and CTO-10Avp column oven. Data acquisition, peak integration and calculation were accomplished with LabSolutions software. A 4.6 13 x 150 mm Atlantis T3 5 micron column was used for fractionation using a gradient mobile phase consisting of solvents A and B with ratios of 5/95/0.1 and 95/5/0.1 acetonitrile/water/TFAacid, respectively, at 50 -95% B in four minutes, 95% B for 2 min, 95 – 50% B in 0.5 min, and 50% B for 4.5 min. Flow rate was 1 mL/min at 45°C. The injection volume was 40 microliters with detection at 325 nm. Quantiation was versus external standards prepared in blank plasma over a linear range of 25 – 750 ng/mL (R2 > 0.995) Extraction of plasma samples was conducted on 100 µL of the respective samples using 0.5 mL of MtBE after adding 10 µL of AI-10-49 spiking solution (10X), vortexing, and allowing the sample sit at room temperature for 5 min. The two phase mixture was vortexed for five min then centrifuged at 12,000 rpm for five min. 450 µL of the MtBE layer was transferred to clean tubes and evaporated to dryness. The resulting residue was reconstituted in 100 µL of 50/50/0.1, ACN/H2O/TFA and vortexed followed by centrifugation at 12,000 rpm for five min. 90 µL of the supernatant was transferred to autosampler vials with polypropylene inserts and analyzed. |
| 毒性/毒理 (Toxicokinetics/TK) |
Although we have not assessed toxicity after longterm exposure, after 7 days of administration of AI-10-49, we observe no evidence of toxicity (figs. S9 to S11).
Toxicology Studies [1] The cumulative toxicity of AI-10-49 was evaluated after daily administration for seven days. These studies did not include maximum tolerated dose or long term (> 30 days) toxicity. Six week old C57BL/6 female mice were treated with daily intraperitoneal injection of DMSO or 200mg/kg AI-10-49 for seven days (n=3 to 4 per group). The appearance of mice was analyzed for signs of toxicity, including grooming, motility, and weight. Four hours after last injection, peripheral blood cells were analyzed by flow cytometry and quantified with hemocytometer. Mice were then euthanized for tissue harvesting. Bone marrow hematopoietic progenitors were analyzed by flow cytometry, including hematopoietic stem and multilineage progenitors [LSK+= Lin(-), kit(+), Sca1(+)], common myeloid progenitors [CMP=Lin(-)Sca1(-)kit(+)CD34(+)CD16/32(-)], granulocyte/monocyte progenitor [GMP=Lin(-)Sca1(-)kit(+)CD34(+)CD16/32(+)], and megakaryocyte/erythroid progenitors [MEP=Lin(-)Sca1(-)kit(+)CD34(-)CD16/32(- )]. Parafin-sections of bone marrow, liver, lung, spleen, intestine, and brain were prepared and stained with hematoxylin & eosin for the analysis of tissue architecture. |
| 参考文献 | |
| 其他信息 |
Acute myeloid leukemia (AML) is the most common form of adult leukemia. The transcription factor fusion CBFβ-SMMHC (core binding factor β and the smooth-muscle myosin heavy chain), expressed in AML with the chromosome inversion inv(16)(p13q22), outcompetes wild-type CBFβ for binding to the transcription factor RUNX1, deregulates RUNX1 activity in hematopoiesis, and induces AML. Current inv(16) AML treatment with nonselective cytotoxic chemotherapy results in a good initial response but limited long-term survival. Here, we report the development of a protein-protein interaction inhibitor, AI-10-49, that selectively binds to CBFβ-SMMHC and disrupts its binding to RUNX1. AI-10-49 restores RUNX1 transcriptional activity, displays favorable pharmacokinetics, and delays leukemia progression in mice. Treatment of primary inv(16) AML patient blasts with AI-10-49 triggers selective cell death. These data suggest that direct inhibition of the oncogenic CBFβ-SMMHC fusion protein may be an effective therapeutic approach for inv(16) AML, and they provide support for transcription factor targeted therapy in other cancers. [1]
|
| 分子式 |
C30H22F6N6O5
|
|
|---|---|---|
| 分子量 |
660.5233
|
|
| 精确质量 |
660.155
|
|
| 元素分析 |
C, 54.55; H, 3.36; F, 17.26; N, 12.72; O, 12.11
|
|
| CAS号 |
1256094-72-0
|
|
| 相关CAS号 |
|
|
| PubChem CID |
49806644
|
|
| 外观&性状 |
Off-white to yellow solid powder
|
|
| 密度 |
1.5±0.1 g/cm3
|
|
| 沸点 |
790.3±70.0 °C at 760 mmHg
|
|
| 闪点 |
431.8±35.7 °C
|
|
| 蒸汽压 |
0.0±2.8 mmHg at 25°C
|
|
| 折射率 |
1.611
|
|
| LogP |
7
|
|
| tPSA |
129.29
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
15
|
|
| 可旋转键数目(RBC) |
12
|
|
| 重原子数目 |
47
|
|
| 分子复杂度/Complexity |
913
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
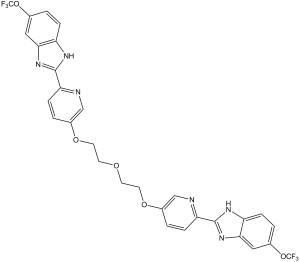
FC(OC1C([H])=C([H])C2=C(C=1[H])N([H])C(C1C([H])=C([H])C(=C([H])N=1)OC([H])([H])C([H])([H])OC([H])([H])C([H])([H])OC1=C([H])N=C(C([H])=C1[H])C1=NC3C([H])=C([H])C(=C([H])C=3N1[H])OC(F)(F)F)=N2)(F)F
|
|
| InChi Key |
WJBSSBFGPKTMQQ-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C30H22F6N6O5/c31-29(32,33)46-17-1-5-21-25(13-17)41-27(39-21)23-7-3-19(15-37-23)44-11-9-43-10-12-45-20-4-8-24(38-16-20)28-40-22-6-2-18(14-26(22)42-28)47-30(34,35)36/h1-8,13-16H,9-12H2,(H,39,41)(H,40,42)
|
|
| 化学名 |
6-(trifluoromethoxy)-2-[5-[2-[2-[6-[6-(trifluoromethoxy)-1H-benzimidazol-2-yl]pyridin-3-yl]oxyethoxy]ethoxy]pyridin-2-yl]-1H-benzimidazole
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (3.78 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (3.78 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 10% DMSO +90%PEG400: 30mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5140 mL | 7.5698 mL | 15.1396 mL | |
| 5 mM | 0.3028 mL | 1.5140 mL | 3.0279 mL | |
| 10 mM | 0.1514 mL | 0.7570 mL | 1.5140 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|---|
|