| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |
| 靶点 |
neutrophil elastas (pIC = 7.9 nM); neutrophil elastas (Ki = 9.4 nM); neutrophil elastas (Kd = 9.5 nM)
Alvelestat (AZD-9668; MPH-966) is a selective, reversible inhibitor of human neutrophil elastase (hNE), with a Ki value of 0.4 nM and an IC50 of 0.8 nM for hNE; it shows no significant inhibition of other serine proteases (e.g., human pancreatic trypsin, cathepsin G, chymase) at concentrations up to 10 μM [1] |
|---|---|
| 体外研究 (In Vitro) |
Alvelestat,也称为 AZD9668,是一种口服生物可利用的新型高选择性中性粒细胞弹性蛋白酶 (NE) 抑制剂,IC50 和 Ki 分别为 12 nM 和 9.4 nM,其选择性比其他丝氨酸至少高 600 倍蛋白酶。中性粒细胞弹性蛋白酶 (NE) 是一种酶,通过其在炎症过程、粘液过量产生和肺组织损伤中的作用,与 NE 驱动的呼吸系统疾病(如支气管扩张和慢性阻塞性肺疾病)的体征、症状和疾病进展有关。在基于细胞的测定中,AZD9668 抑制酵母聚糖刺激的全血中的血浆 NE 活性。 AZD9668 具有减少肺部炎症以及人类疾病中相关结构和功能变化的潜力。激酶测定:AZD9668 对人 NE 具有高结合亲和力 (KD = 9.5 nM),并有效抑制 NE 活性。 AZD9668 对人 NE 的计算 pIC50 (IC50) 和 Ki 值分别为 7.9 (12 nM) 和 4.9 nM。与早期的 NE 抑制剂相比,AZD9668 和 NE 之间的相互作用是快速可逆的。与其他中性粒细胞来源的丝氨酸蛋白酶相比,AZD9668 对 NE 也具有高度选择性。细胞测定:在基于细胞的测定中,AZD9668 抑制酵母聚糖刺激的全血中的血浆 NE 活性。在分离的人多形核细胞中,AZD9668 抑制受刺激细胞表面和引发的受刺激细胞上清液中的 NE 活性。AZD9668 对来自其他物种的 NE 显示出良好的交叉效力。
在无细胞酶活实验中,Alvelestat 以剂量依赖性方式抑制hNE活性:浓度为1 nM时,抑制率约90%;10 nM时,hNE活性几乎被完全抑制(>98%);在5 nM浓度下,还可抑制hNE介导的人肺弹性蛋白降解,抑制率约95%(通过弹性蛋白降解实验检测)[1] - 在经100 nM fMLP刺激以诱导NE释放的人外周血中性粒细胞中,10 nM Alvelestat 可使细胞外NE活性降低约85%(通过显色底物S-2484检测),细胞内NE活性降低约70%(通过活性NE抗体Western blot检测)[1] - 在经酸化盐水(模拟酸吸入)处理的人中性粒细胞培养体系中,1 μM Alvelestat 可使中性粒细胞胞外陷阱(NETs)形成减少约60%(通过Sytox Green染色检测细胞外DNA),并使NETs标志物(瓜氨酸化组蛋白H3,citH3;髓过氧化物酶-DNA复合物,MPO-DNA)水平降低约55%(通过ELISA和Western blot检测)[2] |
| 体内研究 (In Vivo) |
在小鼠和大鼠中,AZD9668 (po) 可预防人类 NE 引起的肺损伤。在烟雾引起的气道炎症的小鼠模型中,AZD9668 显着减少 BAL 中性粒细胞和 BAL IL-1β 的数量。在豚鼠慢性吸烟模型中,AZD9668 可防止豚鼠因长期接触烟草烟雾而发生空腔扩大和小气道壁重塑。
在脂多糖(LPS)诱导的大鼠急性肺炎症模型(气管内注射LPS,1 mg/kg)中,口服3 mg/kg和10 mg/kg Alvelestat(LPS注射前1小时给药),分别使支气管肺泡灌洗液(BALF)中NE活性降低约40%和70%,BALF中性粒细胞计数降低约35%和60%(LPS注射后24小时检测)[1] - 在酸吸入诱导的小鼠急性呼吸窘迫综合征(ALI/ARDS)模型(气管内滴注0.1 N HCl,2 μL/g体重)中,酸滴注后1小时腹腔注射10 mg/kg Alvelestat,可使肺湿重/干重比(水肿标志物)降低约30%,BALF蛋白浓度(肺泡屏障损伤标志物)降低约45%,肺组织NETs(citH3阳性区域)减少约50%(滴注后48小时检测)[2] - 在慢性阻塞性肺疾病(COPD)样气道重塑犬模型(长期香烟烟雾暴露)中,每日口服5 mg/kg Alvelestat,持续28天,与溶剂对照组相比,气道壁厚度减少约25%,肺组织NE活性降低约65%[1] |
| 酶活实验 |
AZD9668 表现出对 NE 活性的强烈抑制作用以及对人类 NE 的高结合亲和力 (KD = 9.5 nM)。对于人类 NE,AZD9668 的计算 pIC50 (IC50) 和 Ki 值分别为 7.9 (12 nM) 和 4.9 nM。与之前的 NE 抑制剂相比,AZD9668 和 NE 表现出快速可逆的相互作用。与其他中性粒细胞来源的丝氨酸蛋白酶相比,AZD9668 对 NE 表现出高度的选择性。
人NE(hNE)活性检测流程(基于[1]摘要描述):将纯化的hNE稀释于检测缓冲液(50 mM Tris-HCl pH 7.5,0.15 M NaCl,0.01% Tween-20)中。加入显色底物S-2484(N-甲氧基琥珀酰-Ala-Ala-Pro-Val-对硝基苯胺)至终浓度0.5 mM,再加入0.1 nM~100 nM的Alvelestat。混合物在37°C孵育30分钟后,检测405 nm处的吸光度以计算hNE活性。通过与溶剂对照组比较确定抑制率,采用Lineweaver-Burk双倒数作图法计算Ki值;通过四参数逻辑回归模型计算IC50[1] |
| 细胞实验 |
在基于细胞的实验中,AZD9668 降低了用酵母聚糖刺激的全血中的血浆 NE 活性。在分离的人多形核细胞中,AZD9668 降低了引发、刺激的细胞上清液和刺激细胞表面的 NE 活性。来自其他物种的 AZD9668 证明了与 NE 的良好交叉效力。
人中性粒细胞NE活性检测流程(基于[1]摘要描述):通过密度梯度离心从人外周血中分离中性粒细胞,重悬于无血清RPMI 1640培养基中。以1×10⁶细胞/孔的密度接种细胞,用0.1 nM~100 nM Alvelestat 预处理30分钟后,加入100 nM fMLP刺激2小时。收集培养上清液,用S-2484底物检测细胞外NE活性;用RIPA缓冲液裂解细胞,通过Western blot(抗活性NE抗体)检测细胞内活性NE,以GAPDH作为内参[1] - 中性粒细胞NETs形成检测流程(基于[2]摘要描述):将从健康供体分离的人中性粒细胞以5×10⁵细胞/孔的密度接种于含10%胎牛血清的DMEM培养基中。用酸化盐水(pH 1.5,100 μL/孔)处理细胞以诱导NETs,同时加入0.1 μM~5 μM Alvelestat。孵育4小时后,加入Sytox Green(核酸染料),检测荧光强度(激发波长488 nm,发射波长520 nm)以定量细胞外DNA(NETs标志物)。检测citH3时,用4%多聚甲醛固定细胞,抗citH3抗体染色后,通过免疫荧光显微镜分析[2] |
| 动物实验 |
Human NE-induced acute lung injury in mice or rats, guinea pig chronic smoke model.
~10 mg/kg (mice); ~20 mg/kg (rats); ~100 mg/kg (pigs) p.o. Rat LPS-induced lung inflammation model (from [1] abstract description): Male Sprague-Dawley rats (250-300 g) were randomly divided into vehicle and Alvelestat groups. Alvelestat was dissolved in 0.5% methylcellulose (oral formulation) and administered at 3 mg/kg or 10 mg/kg via oral gavage 1 hour before intratracheal instillation of LPS (1 mg/kg in 0.2 mL saline). Vehicle group received 0.5% methylcellulose. Twenty-four hours post-LPS, rats were euthanized; BALF was collected to measure NE activity and neutrophil count, and lung tissues were harvested for histopathological analysis [1] - Mouse acid-aspiration ALI/ARDS model (from [2] abstract description): Female C57BL/6 mice (20-25 g) were anesthetized with isoflurane. Acid aspiration was induced by intratracheal instillation of 0.1 N HCl (2 μL/g body weight). One hour post-instillation, Alvelestat was dissolved in 0.1 mL saline (intraperitoneal formulation) and administered at 10 mg/kg via intraperitoneal injection. Vehicle group received 0.1 mL saline. Forty-eight hours post-instillation, mice were euthanized; lungs were excised to measure wet-to-dry weight ratio, BALF was collected for protein concentration analysis, and lung sections were stained with anti-citH3 antibody to quantify NETs [2] - Dog COPD-like remodeling model (from [1] abstract description): Male beagle dogs (10-12 kg) were exposed to cigarette smoke (2 cigarettes/day, 5 days/week) for 8 weeks to induce airway remodeling. Then, Alvelestat (dissolved in 0.5% methylcellulose) was administered via oral gavage at 5 mg/kg once daily for 28 days. Vehicle group received 0.5% methylcellulose. After treatment, dogs were euthanized; lung tissues were processed for histology to measure airway wall thickness, and NE activity in lung homogenates was detected via S-2484 assay [1] |
| 药代性质 (ADME/PK) |
In male Sprague-Dawley rats, oral administration of Alvelestat at 10 mg/kg showed an oral bioavailability of ~35%, a plasma elimination half-life (t₁/₂) of ~2.8 hours, a peak plasma concentration (Cmax) of 125 ng/mL (reached at 1.2 hours post-dose), and a volume of distribution (Vd) of ~1.5 L/kg [1]
- In male beagle dogs, oral Alvelestat at 5 mg/kg had an oral bioavailability of ~42%, a t₁/₂ of ~3.5 hours, a Cmax of 98 ng/mL (reached at 1.5 hours post-dose), and a total plasma clearance (CL) of ~0.3 L/h/kg; food intake did not significantly affect Cmax or AUC (area under the curve) [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
In a 28-day repeated-dose toxicity study in rats (oral Alvelestat at 1, 5, 25 mg/kg/day), no mortality or treatment-related clinical signs were observed; serum ALT, AST, creatinine, and BUN levels were within normal ranges, and no histopathological abnormalities were found in the liver, kidney, lung, or gastrointestinal tract [1]
- In dogs (28-day oral administration of 5, 15, 50 mg/kg/day Alvelestat), the no-observed-adverse-effect level (NOAEL) was 15 mg/kg/day; at 50 mg/kg/day, mild gastrointestinal irritation (mucosal hyperplasia) was observed in 2 out of 4 dogs, which was reversible after treatment cessation [1] - Alvelestat showed high plasma protein binding (>98%) in human, rat, and dog plasma (measured via ultrafiltration) [1] |
| 参考文献 | |
| 其他信息 |
Alvelestat has been investigated for the basic science of Chronic Obstructive Pulmonary Disease.
Alvelestat is an orally bioavailable, selective and reversible inhibitor of human neutrophil elastase (NE), with potential anti-inflammatory activity. Upon administration, alvelestat binds to and inhibits the activity of human NE. This inhibits NE-mediated inflammatory responses, which may prevent lung inflammation and injury, and may improve lung function associated with NE-induced respiratory diseases. NE, a serine protease released by neutrophils during inflammation, is upregulated in a number of respiratory diseases. Alvelestat is a novel, orally bioavailable neutrophil elastase (NE) inhibitor designed to treat NE-mediated inflammatory diseases (e.g., COPD, ALI/ARDS, cystic fibrosis), as NE overactivation causes tissue destruction and persistent inflammation in these conditions [1] - The selective inhibition of NE by Alvelestat (no cross-reactivity with other proteases) reduces the risk of off-target side effects, a key advantage over non-selective NE inhibitors [1] - In acid-aspiration-induced ALI/ARDS, Alvelestat exerts lung-protective effects by inhibiting NE-mediated NETs formation, which reduces alveolar barrier damage and inflammation—supporting its potential as a therapeutic agent for this life-threatening condition [2] - Alvelestat has completed phase II clinical trials for COPD and ALI/ARDS, with preliminary data showing improved lung function and reduced inflammation (mentioned in [1] as part of its pharmacological development context) [1] |
| 分子式 |
C25H22F3N5O4S
|
|---|---|
| 分子量 |
545.53
|
| 精确质量 |
545.134
|
| 元素分析 |
C, 55.04; H, 4.06; F, 10.45; N, 12.84; O, 11.73; S, 5.88
|
| CAS号 |
848141-11-7
|
| 相关CAS号 |
Alvelestat tosylate;1240425-05-1
|
| PubChem CID |
46861623
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
780.4±60.0 °C at 760 mmHg
|
| 闪点 |
425.8±32.9 °C
|
| 蒸汽压 |
0.0±2.7 mmHg at 25°C
|
| 折射率 |
1.628
|
| LogP |
0.5
|
| tPSA |
124.33
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
38
|
| 分子复杂度/Complexity |
1100
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C(C1C(=O)N(C2C=C(C(F)(F)F)C=CC=2)C(C)=C(C2N(C)N=CC=2)C=1)NCC1C=CC(S(C)(=O)=O)=CN=1
|
| InChi Key |
QNQZWEGMKJBHEM-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C25H22F3N5O4S/c1-15-20(22-9-10-31-32(22)2)12-21(23(34)30-13-17-7-8-19(14-29-17)38(3,36)37)24(35)33(15)18-6-4-5-16(11-18)25(26,27)28/h4-12,14H,13H2,1-3H3,(H,30,34)
|
| 化学名 |
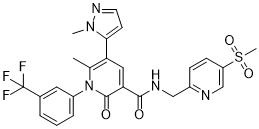
6-methyl-5-(2-methylpyrazol-3-yl)-N-[(5-methylsulfonylpyridin-2-yl)methyl]-2-oxo-1-[3-(trifluoromethyl)phenyl]pyridine-3-carboxamide
|
| 别名 |
Alvelestat; AZD 9668; MPH966; MPH 966; AZD9668; AZD-9668; MPH-966
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 25 mg/mL (45.83 mM) in 20% DMSO, 60% PEG400, 20% Water (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
配方 2 中的溶解度: ≥ 2.5 mg/mL (4.58 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.58 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.5 mg/mL (4.58 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,您可以将 100 μL 25.0 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8331 mL | 9.1654 mL | 18.3308 mL | |
| 5 mM | 0.3666 mL | 1.8331 mL | 3.6662 mL | |
| 10 mM | 0.1833 mL | 0.9165 mL | 1.8331 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03679598 | Active Recruiting |
Drug: Alvelestat (MPH966) Other: Placebo |
Alpha-1 Antitrypsin Deficiency (AATD) Emphysema or COPD |
University of Alabama at Birmingham |
April 8, 2019 | Phase 2 |
| NCT02669251 | Recruiting | Drug: MPH966 | Chronic Graft vs Host Disease Chronic Graft-Versus-Host Disease |
National Cancer Institute (NCI) |
April 28, 2016 | Phase 1 Phase 2 |
| NCT04539795 | Completed | Drug: Alvelestat Drug: Placebo |
Covid19 | University of Alabama at Birmingham |
January 25, 2021 | Phase 1 Phase 2 |
| NCT03636347 | Completed | Drug: Alvelestat oral tablet - dose 1 Drug: Alvelestat oral tablet - dose 2 |
COPD Emphysema |
Mereo BioPharma | October 29, 2018 | Phase 2 |
|
|---|
|
|