| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 靶点 |
Serine Protease; Granzyme; I-kappaBalpha
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:Nafamostat 甲磺酸盐在 60 分钟和 120 分钟时显着抑制血小板 β-血栓球蛋白 (β TG) 的释放。甲磺酸萘莫司他 (NM) 可防止中性粒细胞弹性蛋白酶的显着释放; 120 分钟时,NM 组血浆弹性蛋白酶-α 1-抗胰蛋白酶复合物为 0.16 mg/mL,对照组为 1.24 mg/mL。 Nafamostat mesilate 完全抑制 C1 抑制剂与激肽释放酶和 FXIIa 形成复合物。 Nafamostat mesilate 抑制多种蛋白酶,这些蛋白酶可能在弥散性血管内凝血 (DIC) 的病理生理学中发挥重要作用。Nafamostat mesilate 以浓度依赖性方式抑制外在途径活性(TF-F.VIIa 介导的 F.Xa 生成),IC50 为 0.1 μM 。 Nafamostat mesilate 对双相 ASIC3 电流的初始相瞬态分量产生浓度依赖性抑制,IC50 值约为 2.5 mM。细胞测定:在通过ELISA评估NF-κB活化时,联合组PANC-1细胞核提取物中NF-κB p65的浓度在统计学上低于奥沙利铂组(p<0.0001)。与核 NF-κB 水平一样,Western blot 分析显示联合组磷酸化 IκBa 水平显着低于奥沙利铂组 (p=0.037)。换句话说,FUT-175 在体外通过抑制 IκBa 磷酸化来抑制奥沙利铂诱导的 NF-κB 激活。
|
| 体内研究 (In Vivo) |
Nafamostat mesilate (10 mg/kg) 抑制类胰蛋白酶诱导的抓挠,但不抑制组胺和血清素诱导的抓挠。 Nafamostat mesilate (1-10 mg/kg) 对皮内化合物 48/80(10 mg/位点)引起的抓挠产生剂量依赖性抑制。 Nafamostat mesilate (10 mg/kg) 可抑制小鼠皮肤中的类胰蛋白酶活性。 Nafamostat mesilate 抑制吉西他滨诱导的 NF-κB 激活,增强吉西他滨引起的细胞凋亡并抑制胰腺肿瘤生长。甲磺酸萘莫司他联合吉西他滨可改善吉西他滨引起的小鼠体重减轻。
|
| 酶活实验 |
炎症中体液和细胞参与者的激活会增加体外循环术后出血和多器官损伤的风险。我们现在在模拟体外循环的体外回路中比较单独使用肝素与甲磺酸那法莫司他酯(NM)的效果,后者是一种具有胰蛋白酶样酶特异性的蛋白酶抑制剂。NM在60分钟和120分钟时显著抑制血小板β-血栓球蛋白(β-TG)的释放。血小板计数没有差异。ADP诱导的NM回路聚集减少,这是由于NM对血小板功能的直接影响。NM可防止中性粒细胞弹性蛋白酶的任何显著释放;在120分钟时,NM组的血浆弹性蛋白酶α1-抗胰蛋白酶复合物为0.16微克/毫升,对照组为1.24微克/毫升。NM完全抑制C1抑制剂与激肽释放酶和FXIIa复合物的形成。NM不改变补体激活的标志物(C1-C1抑制剂复合物和C5b-9)或凝血酶形成的指标(F1.2)。然而,在120分钟时,通过纤维蛋白肽A的释放测量的凝血酶活性显著降低。数据表明,CPB期间的补体激活与中性粒细胞激活相关性较差,激肽释放酶或FXIIa或两者都可能是更重要的激动剂。NM抑制两种重要接触系统蛋白以及血小板和中性粒细胞释放的能力增加了在临床CPB期间抑制炎症反应的可能性[1]。
|
| 细胞实验 |
细胞活力测定[8]
细胞类型:MDAPanc-28细胞 测试浓度:80μg/mL 培养时间:24小时、48小时 实验结果:在24小时和48小时显著降低MDAPanc-28细胞的细胞活力。 吉西他滨目前是胰腺癌的标准一线化疗药物。然而,由于吉西他滨诱导的核因子- kappab (NF-kappaB)活化,吉西他滨出现了化学耐药。我们之前报道了合成丝氨酸蛋白酶抑制剂Nafamostat mesilate抑制NF-kappaB激活并诱导胰腺癌细胞凋亡。本研究探讨Nafamostat mesilate是否能增强吉西他滨的抗癌作用。 材料和方法:通过电泳迁移位移法(体外)和免疫组化法(体内)研究p65在癌细胞中的位置,检测不同药物处理胰腺癌细胞中NF-kappaB的活化情况。流式细胞术检测药物对肿瘤细胞凋亡的影响。 结果:Nafamostat mesilate抑制吉西他滨诱导的NF-kappaB活化,增强吉西他滨诱导的细胞凋亡,抑制胰腺肿瘤生长。有趣的是,联合治疗改善了吉米他滨诱导的小鼠体重减轻。 结论:这种联合化疗可能是治疗胰腺癌的潜在新策略。[5] |
| 动物实验 |
Male ICR-SCID nude mice
30 mg/kg i.p. Nafamostat mesilate was dissolved in 5% glucose and was injected intravenously 5 min before pruritogen injection. The skin was isolated from the murine back 5 min after nafamostat administration and the activities of tryptase and chymase in the skin were determined, according to the method described by Wolters et al. (2001). For the assay of tryptase activity, the skin sample was homogenized and sonicated in 10 mM TRIS (tris(hydroxymethyl)aminomethane), pH 6.1, containing 2 M NaCl. The solution was centrifuged at 700×g for 5 min at 4 °C. One microliter of the supernatant (5 mg protein/ml) was added to 49 μl of solution A (0.06 M TRIS, pH 7.8, containing 0.4% dimethyl sufoxide and 30 μg/ml heparin). The cocktail (50 μl) was reacted with 50 μl of 480 μg/ml N-p-Tosyl-Gly-Pro-Arg-p-nitroanilide in solution A at 37 °C for 1 h. Free nitroaniline released was measured colorimetrically at 420 nm. For the assay of chymase activity, skin sample was homogenized and sonicated in solution B (0.45 M TRIS, pH 8.0, containing 0.1% dimethyl sufoxide and 1.8 mM NaCl). The homogenate was centrifuged at 700×g for 5 min at 4 °C. Ten microliters of the supernatant (5 mg protein/ml) was added to 40 μl of solution B. This cocktail (50 μl) was reacted with 50 μl of 2 mg/ml succinyl-Ala-Ala-Pro-Phr-p-nitroanilide acetate in solution B at 37 °C for 1 h. Free nitroaniline released was measured colorimetrically at 420 nm.[4] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Two metabolites of NM, p-guanidinobenzoic acid (PGBA) and 6-amidino-2-naphthol (AN), are renally excreted. Nafamostat accumulates in the kidneys. Metabolism / Metabolites Nafamostat is mainly hydrolyzed by hepatic carboxyesterase and long-chain acyl-CoA hydrolase in human liver cytosol. Main metabolites are p-guanidinobenzoic acid (PGBA) and 6-amidino-2-naphthol (AN) as inactive protease inhibitors. Biological Half-Life Approximately 8 minutes |
| 参考文献 |
[1]. Thromb Haemost . 1996 Jan;75(1):76-82. [2]. Thromb Res . 1994 Apr 15;74(2):155-61. [3]. Biochem Biophys Res Commun . 2007 Nov 9;363(1):203-8. [4]. Eur J Pharmacol . 2006 Jan 13;530(1-2):172-8. [5]. Anticancer Res . 2009 Aug;29(8):3173-8. [6]. Antimicrob Agents Chemother . 2020 May 21;64(6):e00754-20. [8]. Cancer: Interdisciplinary International Journal of the American Cancer Society, 2007, 109(10): 2142-2153. |
| 其他信息 |
Nafamostat Mesylate is the mesylate salt form of nafamostat, a broad-spectrum, synthetic serine protease inhibitor, with anticoagulant, anti-inflammatory, mucus clearing, and potential antiviral activities. Upon administration, nafamostat inhibits the activities of a variety of proteases, including thrombin, plasmin, kallikrein, trypsin, and Cl esterase in the complement system, and factors VIIa, Xa, and XIIa in the coagulation system. Although the mechanism of action of nafamostat is not fully understood, trypsinogen activation in the pancreas is known to be a trigger reaction in the development of pancreatitis. Nafamostat blocks the activation of trypsinogen to trypsin and the inflammatory cascade that follows. Nafamostat may also decrease epithelial sodium channel (ENaC) activity and increase mucus clearance in the airways. ENaC activity is increased in cystic fibrosis. In addition, nafamostat may inhibit the activity of transmembrane protease, serine 2 (TMPRSS2), a host cell serine protease that mediates viral cell entry for influenza virus and coronavirus, thereby inhibiting viral infection and replication.
The pruritogenic potency of tryptase and its involvement in anti-pruritic effect of intravenous nafamostat mesilate (NFM) were studied in mice. An intradermal injection of tryptase (0.05-1 ng/site) elicited scratching in ICR mice, while chymase was without effects at doses of 0.05-50 ng/site. The dose-response curve of tryptase action was bell-shaped and the effect peaked at 0.1 ng/site (approximately 0.7 fmol/site). NFM (10 mg/kg) inhibited scratching induced by tryptase but not by histamine and serotonin. NFM (1-10 mg/kg) produced the dose-dependent inhibition of scratching induced by intradermal compound 48/80 (10 microg/site). The inhibition by NFM (10 mg/kg) was abolished in mast cell-deficient (WBB6F1 W/W(V)) mice, but not in wild-type (WBB6F1 +/+) mice. NFM (10 mg/kg) suppressed tryptase activity in the mouse skin. Proteinase-activated receptor-2 (PAR-2) neutralizing antibody (0.1 and 1 microg/site) and the PAR-2 antagonist FSLLRY (10 and 100 microg/site) inhibited scratching induced by tryptase (0.1 ng/site) and compound 48/80 (10 microg/site). These results suggest that mast cell tryptase elicits itch through PAR-2 receptor and that NFM inhibits itch-associated responses mainly through the inhibition of mast cell tryptase.[4] |
| 分子式 |
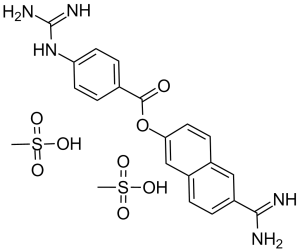
C21H25N5O8S2
|
|
|---|---|---|
| 分子量 |
539.58
|
|
| 精确质量 |
539.11445512
|
|
| 元素分析 |
C, 46.75; H, 4.67; N, 12.98; O, 23.72; S, 11.88
|
|
| CAS号 |
82956-11-4
|
|
| 相关CAS号 |
Nafamostat;81525-10-2;Nafamostat hydrochloride;80251-32-7; 82956-11-4 (mesylate)
|
|
| PubChem CID |
5311180
|
|
| 外观&性状 |
Off-white to light yellow solid powder
|
|
| 沸点 |
637.2ºCat 760 mmHg
|
|
| 熔点 |
259-261°C
|
|
| 闪点 |
339.1ºC
|
|
| LogP |
4.906
|
|
| tPSA |
200.82
|
|
| 氢键供体(HBD)数目 |
6
|
|
| 氢键受体(HBA)数目 |
10
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
36
|
|
| 分子复杂度/Complexity |
645
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O=C(C1C=CC(NC(N)=N)=CC=1)OC1C=C2C(C=C(C(N)=N)C=C2)=CC=1.O=S(C)(O)=O
|
|
| InChi Key |
SRXKIZXIRHMPFW-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C19H17N5O2.2CH4O3S/c20-17(21)14-2-1-13-10-16(8-5-12(13)9-14)26-18(25)11-3-6-15(7-4-11)24-19(22)23;2*1-5(2,3)4/h1-10H,(H3,20,21)(H4,22,23,24);2*1H3,(H,2,3,4)
|
|
| 化学名 |
(6-carbamimidoylnaphthalen-2-yl) 4-(diaminomethylideneamino)benzoate;methanesulfonic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.63 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.63 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.63 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8533 mL | 9.2665 mL | 18.5329 mL | |
| 5 mM | 0.3707 mL | 1.8533 mL | 3.7066 mL | |
| 10 mM | 0.1853 mL | 0.9266 mL | 1.8533 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT06078839 | Not yet recruiting | Drug: Nafamostat mesilate Drug: 5% glucose |
Sepsis Nafamostat Mesilate |
Xu Li | October 1, 2023 | Phase 4 |
| NCT05555641 | Recruiting | Drug: Nafamostat Mesylate Drug: Unfractionated Heparin |
Critical Illness Anticoagulation |
Xiaobo Yang, MD | December 20, 2022 | Phase 2 |
| NCT05090280 | Active Recruiting |
Drug: Nafamostat Mesylate Drug: PF614 solution |
Pharmacokinetics | Ensysce Biosciences | December 1, 2021 | Phase 1 |
| NCT04483960 | Recruiting | Drug: Nafamostat Mesilate Drug: Enoxaparin |
SARS-CoV-2 Infection (COVID-19) |
University of Melbourne | July 28, 2020 | Phase 3 |
| NCT04473053 | Recruiting | Drug: Nafamostat Mesylate Drug: TD139 |
COVID-19 | University of Edinburgh | July 3, 2020 | Phase 1 Phase 2 |