| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g | |||
| Other Sizes |
| 靶点 |
D2 Receptor ( IC50 = 2.8 nM ); D3 Receptor ( IC50 = 3.2 nM )
|
|
|---|---|---|
| 体外研究 (In Vitro) |
体外活性:氨磺必利对克隆并稳定转染的人多巴胺 D2 表现出高亲和力,对于 D2 和 D3 受体亚型的 Ki 值分别为 2.8±0.4nM 和 3.2±0.3nM。据报道,Amisulpride 可抑制放射性配体与大鼠纹状体膜中天然多巴胺 D2 受体的结合,IC50 值为 21nM。此外,Amisulpride 在大鼠边缘系统中可取代体内 [3H]raclopride 结合,ED50 值为 17.3±1.86mg/kg。此外,Amisulpride 可抑制喹吡罗诱导的 [3H]胸苷,IC50 值为 22±3nM。细胞测定:评估氨磺必利对多巴胺 D3 受体亚型的功能作用。简而言之,通过在 1 μM 毛喉素存在下添加 10 nM 喹吡罗,在用人多巴胺 D3 受体 cDNA 稳定转染的 NG108-15 神经母细胞瘤-神经胶质瘤细胞中引发的促有丝分裂反应通过[3H]胸苷的掺入进行定量。在氨磺必利浓度增加(0.1 至 100 nM)的情况下测量喹吡罗诱导的有丝分裂的拮抗作用。
|
|
| 体内研究 (In Vivo) |
只有最高剂量的氨磺必利(100 mg/kg)才能显着降低纹状体或边缘系统中的多巴胺水平。 Amisulpride 在 20 和 100 mg/kg 剂量下显着增加大鼠纹状体和边缘系统中多巴胺的合成。氨磺必利(0.5至75毫克/千克)不会引起纹状体中多巴积累的额外增加,但在75毫克/千克时略微加速边缘系统中多巴胺的合成。与媒介物处理的对照组相比,Amisulpride (10 mg/kg) 增加细胞外多巴胺水平。给予阿米磺必利(皮下注射0.5至15毫克/千克)会引起刺激引起的多巴胺释放呈时间和剂量依赖性增加。氨磺必利在 30 和 100 mg/kg 剂量下显着降低纹状体 ACh 水平(分别为对照水平的 87.5% 和 56.3%)[1]。在两项急性研究中,氨磺必利(70 mg/kg,口服)显着增加游泳行为的持续时间[F(3,28)=45.90,p<0.01]。
|
|
| 细胞实验 |
评估氨磺必利对多巴胺 D3 受体亚型的功能作用。总之,[3H]胸苷掺入测量了通过添加 10 nM 喹吡罗稳定转染人多巴胺 D3 受体 cDNA 的 NG108-15 神经母细胞瘤-神经胶质瘤细胞中诱导的有丝分裂反应在 1 μM 毛喉素存在下。当氨磺必利浓度从 0.1 nM 增加至 100 nM 时,可测量喹吡罗诱导的有丝分裂的拮抗作用[1]。
|
|
| 动物实验 |
|
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following oral administration, amisulpride is rapidly absorbed with absolute bioavailability of 48%. Amisulpride has two absorption peaks, with one rapidly achieved within one hour post-dose and a second peak occurring between three to four hours post-dose. Following oral administration of a 50 mg dose, two peak plasma concentrations were 39 ± 3 and 54 ± 4 ng/mL. Following intravenous administration, the peak plasma concentration of amisulpride is achieved at the end of the infusion period and the plasma concentration decreases by 50% within approximately 15 minutes. The AUC(0-∞) increases dose-proportionally in the dose range from 5 mg to 40 mg, which is about four times the maximum recommended dose. In healthy patients receiving intravenous amisulpride, the mean (SD) Cmax was 200 (139) ng/mL at the dose of 5 mg and 451 (230) ng/mL at the dose of 10 mg. The AUC ranged from 136 to 154 ng x h/mL in the dose range of 5 mg to 10 mg. In patients undergoing surgery, the mean (SD) Cmax ranged from 127 (62) to 161 (58) ng/mL at the dose of 5 mg. At the dose of 10 mg, it was 285 (446) ng/mL. The AUC ranged from 204 to 401 ng x h/mL. Following intravenous administration, about 74% of amisulpride is excreted in urine, where 58% of the recovered dose was excreted as unchanged amisulpride. About 23% of the dose is excreted in feces, with 20% of the excreted dose as unchanged parent drug. Following intravenous administration, about four metabolites were identified in urine and feces, accounting for less than 7% of the total dose administered. About 22 to 25% of orally administered amisulpride is excreted in urine, mostly as the unchanged parent drug. Following oral administration, the volume of distribution is 5.8 L/kg. Following intravenous infusion, the mean volume of distribution of amisulpride is estimated to be 127 to 144 L in surgical patients and 171 L in healthy subjects. The plasma clearance of amisulpride is 20.6 L/h in surgical patients and 24.1 L/h in healthy subjects following intravenous administration. Renal clearance was estimated to be 20.5 L/hr (342 mL/min) in healthy subjects. Metabolism / Metabolites Amisulpride undergoes minimal metabolism and its metabolites in plasma are largely undetectable. Two identified metabolites, formed by de-ethylation and oxidation, are pharmacologically inactive and account for approximately 4% of the dose. Metabolites remain largely uncharacterized. Metabolism of amisulpride does not involve cytochrome P450 enzymes. Biological Half-Life Elimination is biphasic. The elimination half-life of amisulpride is approximately 12 hours after an oral dose. The mean elimination half-life is approximately four to five hours in both healthy subjects and patients undergoing surgery receiving intravenous amisulpride. |
|
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Intravenous single doses of amisulpride are generally well tolerated, and in multiple randomized controlled trials were not associated with rates of serum aminotransferase or bilirubin elevations above those that occurred in placebo treated subjects. While oral amisulpride has been linked to transient serum aminotransferase elevations during therapy, single intravenous doses of amisulpride have not been linked to liver enzyme elevations in excess of rates found postoperatively. Since its approval and more widespread use, amisulpride has not been implicated in cases of clinically apparent liver injury. Likelihood score: E (unlikely cause of clinically apparent liver injury). Protein Binding Plasma protein binding ranges from 25% to 30% in the concentration range from 37 to 1850 ng/mL. Amisulpride distributes into erythrocytes. |
|
| 参考文献 |
|
|
| 其他信息 |
Pharmacodynamics
Amisulpride is a selective dopamine D2 and D3 receptor antagonist with no affinity towards other dopamine receptor subtypes. Amisulpride is an atypical antipsychotic agent that works as an antagonist at dopamine receptors in the limbic system. Since it works preferentially in the limbic system, amisulpride is less likely to be associated with extrapyramidal adverse effects than other atypical antipsychotic agents. Amisulpride has no affinity for serotonin, alpha-adrenergic, H1-histamine, cholinergic, and sigma receptors. In clinical trials, amisulpride improved reduced secondary negative symptoms, affective symptoms, and psychomotor retardation in patients with acute exacerbation of schizophrenia. Notably, amisulpride has a differential target binding profile at different doses: at low doses, amisulpride selectively binds to presynaptic dopamine autoreceptors. At high doses, it preferentially binds to post-synaptic dopamine receptors. This explains how amisulpride reduces negative symptoms at low doses and mediates antipsychotic effects at high doses. One study alluded that the antinociceptive effects of amisulpride are mediated through opioid receptor acvitation and D2 receptor antagonism. The actions of amisulpride at opioid receptors may explain its pro-convulsant properties. Amisulpride is also an antiemetic agent that prevents and alleviates postoperative nausea and vomiting. It primarily works by blocking dopamine signalling in the chemoreceptor trigger zone, which is a brain area that relays stimuli to the vomiting center. In clinical trials comprising Caucasian and Japanese subjects, amisulpride caused dose- and concentration-dependent prolongation of the QT interval; thus, intravenous infusion under a strict dosing regimen and close monitoring of patients with pre-existing cardiovascular conditions are recommended. Amisulpride increases plasma prolactin levels, leading to an association with benign pituitary tumours such as prolactinoma. |
| 分子式 |
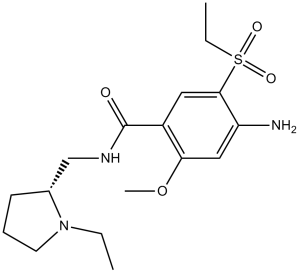
C17H27N3O4S
|
|
|---|---|---|
| 分子量 |
369.48
|
|
| 精确质量 |
369.172
|
|
| 元素分析 |
C, 55.26; H, 7.37; N, 11.37; O, 17.32; S, 8.68
|
|
| CAS号 |
71675-85-9
|
|
| 相关CAS号 |
Amisulpride-d5; 1216626-17-3; Amisulpride hydrochloride; 81342-13-4; Amisulpride-d5 N-Oxide; 1794756-15-2
|
|
| PubChem CID |
2159
|
|
| 外观&性状 |
Solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
558.9±50.0 °C at 760 mmHg
|
|
| 熔点 |
124-128ºC
|
|
| 闪点 |
291.8±30.1 °C
|
|
| 蒸汽压 |
0.0±1.5 mmHg at 25°C
|
|
| 折射率 |
1.546
|
|
| LogP |
1.6
|
|
| tPSA |
110.11
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
7
|
|
| 重原子数目 |
25
|
|
| 分子复杂度/Complexity |
549
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
CCN1C(CNC(C2=CC(S(=O)(CC)=O)=C(N)C=C2OC)=O)CCC1
|
|
| InChi Key |
NTJOBXMMWNYJFB-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C17H27N3O4S/c1-4-20-8-6-7-12(20)11-19-17(21)13-9-16(25(22,23)5-2)14(18)10-15(13)24-3/h9-10,12H,4-8,11,18H2,1-3H3,(H,19,21)
|
|
| 化学名 |
4-amino-N-[(1-ethylpyrrolidin-2-yl)methyl]-5-ethylsulfonyl-2-methoxybenzamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.77 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.77 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: 2.5 mg/mL (6.77 mM) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7065 mL | 13.5325 mL | 27.0651 mL | |
| 5 mM | 0.5413 mL | 2.7065 mL | 5.4130 mL | |
| 10 mM | 0.2707 mL | 1.3533 mL | 2.7065 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Psychobiological Mechanisms Underlying Chronic Pain
CTID: NCT04674670
Phase: N/A Status: Completed
Date: 2023-09-07