| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
AN0128(化合物 2g;10 μM;24-48 小时)在促炎细胞因子(TNF-α、IL-1β)的产生方面对人外周血单核细胞(PBMC)具有有效的抑制作用,但对IFN-γ 或 IL-4 的释放不受抑制[1]。
|
|---|---|
| 体内研究 (In Vivo) |
AN0128(1%、5%;每天局部给药,持续 7 天)可显着抑制炎症浸润的产生,并最大限度地减少骨质流失 [2]。
|
| 动物实验 |
Animal/Disease Models: Experimental periodontitis in 12weeks old male SD (SD (Sprague-Dawley)) rats (weight 275 to 300 g each) [2]
Doses: 1% AN0128 in 40% Transcutol P, 40% PBS and 20% ethanol vehicle Daily topical application for 7 days. Experimental Results: Similar to ketorolac, bone area and bone volume increased by 50% and 35% respectively, and bone loss and inflammation were diminished by 38% and 42% respectively. Animal/Disease Models: Experimental periodontitis in 12weeks old male SD (SD (Sprague-Dawley)) rats (weight 275 to 300 g each) [2] Doses: Use with Total Toothpaste 5% Route of Administration: Apply daily via cotton-tipped applicator . Results for 7 days: better than Total toothpaste, increasing bone mass by 33% and reducing bone loss by 47%. |
| 参考文献 |
|
| 其他信息 |
AN0128 is a novel compound that contains a boron atom within a borinic acid complex. AN0128 has broad spectrum activity against a wide variety of Gram positive bacteria, including many that are known skin colonizers. Of particular importance is P. acnes and its causal role in acne vulgaris. The rise in antibiotic resistance of P. acnes to standard antibiotics necessitates the development of new treatment agents. AN0128 is a good candidate for a topical antibiotic and is currently being developed by Anacor as a novel therapeutic for acne and atopic dermatitis.
Drug Indication Investigated for use/treatment in acne, atopic dermatitis, pediatric indications, and psoriasis and psoriatic disorders. Mechanism of Action AN0128 inhibits the release of pro-inflammatory cytokines, including TNF- alpha, without affecting the normal immune response. |
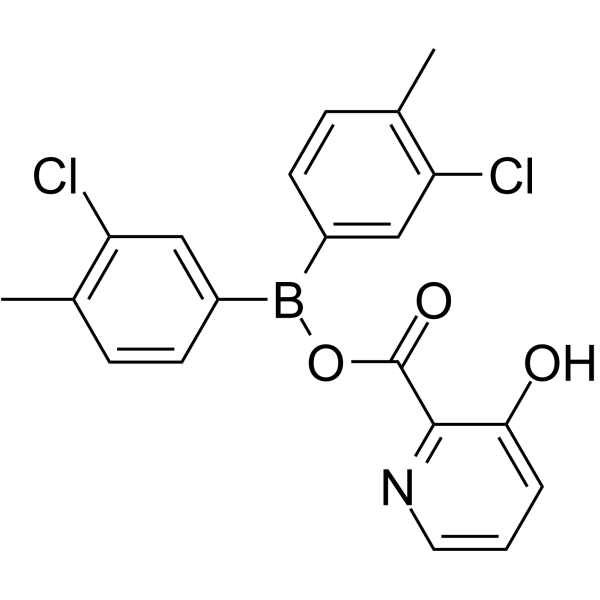
| 分子式 |
C20H16BCL2NO3
|
|---|---|
| 分子量 |
400.062943458557
|
| 精确质量 |
399.06
|
| 元素分析 |
C, 60.05; H, 4.03; B, 2.70; Cl, 17.72; N, 3.50; O, 12.00
|
| CAS号 |
872044-70-7
|
| PubChem CID |
11704019
|
| 外观&性状 |
Solid powder
|
| LogP |
3.673
|
| tPSA |
59.42
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
486
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC1=C(C)C=CC(B(C2C=CC(C)=C(C=2)Cl)OC(C2C(=CC=CN=2)O)=O)=C1
|
| InChi Key |
ZTLSOLUCMQEGEA-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C20H16BCl2NO3/c1-12-5-7-14(10-16(12)22)21(15-8-6-13(2)17(23)11-15)27-20(26)19-18(25)4-3-9-24-19/h3-11,25H,1-2H3
|
| 化学名 |
bis(3-chloro-4-methylphenyl)boranyl 3-hydroxypyridine-2-carboxylate
|
| 别名 |
AN-0128; CRM-0005; ONT-0001; AN0128; CRM0005; ONT0001; AN 0128; CRM 0005; ONT 0001
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~125 mg/mL (~312.45 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (5.20 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.20 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4996 mL | 12.4981 mL | 24.9963 mL | |
| 5 mM | 0.4999 mL | 2.4996 mL | 4.9993 mL | |
| 10 mM | 0.2500 mL | 1.2498 mL | 2.4996 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01288391 | COMPLETED | Drug: NNC 0128-0000-2011 Drug: NNC 0128-0000-2011 |
Congenital Bleeding Disorder Haemophilia A Haemophilia B |
Novo Nordisk A/S | 2011-01 | Phase 1 |
| NCT04728594 | COMPLETEDWITH RESULTS | Behavioral: Social Proof Behavioral: Reframing Behavioral: Scarcity Message |
Communication Vaccination Refusal |
Geisinger Clinic | 2021-01-15 | Not Applicable |
| NCT06337032 | NOT YET RECRUITING | Drug: F/TAF (High Dose Tablet) Drug: F/TAF (Low Dose Tablet) Drug: F/TAF (Lowest Dose Tablet) |
HIV-1-infection | Gilead Sciences | 2024-08 | Phase 4 |
| NCT02518139 | COMPLETEDWITH RESULTS | Drug: TD-4208 Drug: Tiotropium |
Chronic Obstructive Pulmonary Disease (COPD) | Mylan Inc. | 2015-09 | Phase 3 |
| NCT00879229 | TERMINATEDWITH RESULTS | Drug: Ambrisentan Drug: Placebo |
Idiopathic Pulmonary Fibrosis Pulmonary Hypertension |
Gilead Sciences | 2009-07 | Phase 3 |