| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
CYP2C9 (Ki = 2 μM); Natural flavonoid; antioxidative; anti-inflammatory; anti-viral; anti-tumor
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
Ki 是 CYP2C9 RECO 系统(一种包含重组人 CYP2C9、P450 的纯化重组酶系统)中的 2 μM 还原酶、细胞色素 b5 和脂质体[1]。芹菜素 (4',5,7-三羟基黄酮) 抑制细胞色素 P450 2C9 (CYP2C9)。芹菜素抑制细胞增殖。芹菜素在 20、40 和 80 μM 时的第七生长抑制率 (IR) 依次为 38%、71% 和 99%。暴露于芹菜素 24 或 48 小时后,SGC-7901 细胞的克隆形成以依赖于时间和剂量的方式受到抑制。芹菜素处理 24 和 48 小时后,80 μM 的克隆效率分别为 9.8% 和 5%,而对照组的克隆效率为 40.4% 和 43.4% [2]。
|
||
| 体内研究 (In Vivo) |
天然黄酮芹菜素(4',5,7-三羟基黄酮)具有多种生物活性,如抗炎、神经保护、抗癌和抗氧化能力。芹菜素(125 mg/kg 和 250 mg/kg)可减轻阿霉素(ADR)(24 mg/kg)引起的心肌损伤。芹菜素可防止血清天冬氨酸转氨酶 (AST) 的释放。 apelin 可减少血清乳酸脱氢酶 (LDH) 的释放。芹菜素可降低血清肌酸激酶 (CK) 水平 [3]。
|
||
| 酶活实验 |
心肌酶的测定[3]
测定血清天冬氨酸氨基转移酶(AST)、乳酸脱氢酶(LDH)和肌酸激酶(CK),以评估心肌损伤。使用试剂盒测量这些心肌酶。根据制造商对不同试剂盒的说明执行了详细的程序。 |
||
| 细胞实验 |
通过MTT法、克隆形成试验和形态学观察,观察芹菜素对人胃癌SGC-7901细胞生长、克隆形成和增殖的影响。荧光染色和流式细胞术分析用于检测细胞凋亡。
结果:芹菜素明显抑制SGC-7901细胞的生长、克隆形成和增殖,呈剂量依赖性。在80微摩尔/升的浓度下,在第1天观察到生长抑制,而4天后,抑制率(IR)为90%。在20、40和80微摩尔/升的浓度下,第7天的生长IRs分别为38%、71%和99%。用芹菜素处理细胞48小时后,对照组、20、40、80微摩尔/L组的克隆形成数分别为217+/-16.9、170+/-11.1(P<0.05)、98+/-11.1(P>0.05)和25+/-3.5(P<0.05)。荧光染色发现凋亡的典型形态学变化。细胞核失去了光滑的边界,染色质浓缩,细胞核破裂。流式细胞术检测到典型的凋亡峰。用芹菜素处理细胞48小时后,20、40和80微摩尔/升组的凋亡率分别为5.76%、19.17%和29.30%。
结论:芹菜素通过诱导细胞凋亡对SGC-7901细胞的生长和克隆形成具有明显的抑制作用[2]。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Four hours after administration of a flavonoid glycoside extract (corresponding to 0.942 mg aglycones) by gavage, the aglycone of apigenin was observed in the lumen and the wall of the stomach, in the lumen of the small intestine and in the lumen and wall of the cecum in Wistar rats. The evidence of glycosides in the stomach wall suggested that the absorption of flavonoids did not require the presence of their aglycones. Under the study conditions, no renal excretion of apigenin was detected ... Apigenin appears to be absorbable by humans after intake of parsley (Petroselinum crispum). In a randomized crossover study with two one-week intervention periods in succession, fourteen volunteers consumed a diet that included 20 g parsley. The urinary excretion of apigenin was significantly higher (P < 0.05) during the intervention with parsley (20.7 to 5727.3 g/24 hr) than during the basic diet (0 to 1571.7 g/24 hr). The half-life for apigenin was calculated to be on the order of 12 hr. Significant individual variation in the bioavailability and excretion of apigenin was observed ... ... Eleven healthy subjects (5 women, 6 men) in the age range of 23 to 41 years and with an average body mass index of 23.9 + or - 4.1 kg/sq m took part in this study. After an apigenin- and luteolin-free diet, a single oral bolus of 2 g blanched parsley (corresponding to 65.8 + or - 15.5 umol apigenin) per kilogram body weight was consumed. Blood samples were taken at 0, 4, 6, 7, 8, 9, 10, 11 and 28 hr after parsley consumption and 24-hour urine samples were collected ... On average, a maximum apigenin plasma concentration of 127 + or - 81 nmol/L was reached after 7.2 + or - 1.3 hr with a high range of variation between subjects. For all participants, plasma apigenin concentration rose after bolus ingestion and fell within 28 hr under the detection limit (2.3 nmol/L). The average apigenin content in 24-hour urine was 144 + or - 110 nmol/24 hr corresponding to 0.22 + or - 0.16% of the ingested dose. The flavone could be detected in red blood cells without showing dose-response characteristics. ... The present paper shows the study of the absorption and excretion of luteolin and apigenin in rats after a single oral dose of Chrysanthemum morifolium extract (CME) (200 mg/kg). The levels of luteolin and apigenin in plasma, urine, feces, and bile were measured by HPLC after deconjugation with hydrochloric acid or beta-glucuronidase/sulfatase. The results showed that the plasma concentrations of luteolin and apigenin reached the highest peak level at 1.1 and 3.9 hr after dosing, respectively. The area under the concentration-time curves (AUC) for luteolin and apigenin were 23.03 and 237.6 ug h/mL, respectively. The total recovery of the dose was 37.9% (6.6% in urine; 31.3% in feces) for luteolin and 45.2% (16.6% in urine; 28.6% in feces) for apigenin. The cumulative luteolin and apigenin excreted in the bile was 2.05% and 6.34% of the dose, respectively. All of the results suggest apigenin may be absorbed more efficiently than luteolin in CME in rats, and both luteolin and apigenin have a slow elimination phase, with a quick absorption, so a possible accumulation of the two flavonoids in the body can be hypothesized. After a single oral administration of radiolabeled apigenin /to rats/, 51.0% of radioactivity was recovered in urine, 12.0% in feces, 1.2% in the blood, 0.4% in the kidneys, 9.4% in the intestine, 1.2% in the liver, and 24.8% in the rest of the body within 10 days. Sex differences appear with regard to the nature of compounds eliminated via the urinary route: immature male and female rats, like mature female rats, excreted a higher percentage of the mono-glucuronoconjugate of apigenin than the mono-sulfoconjugate of apigenin (10.0 to 31.6% versus 2.0 to 3.6%, respectively). Mature male rats excreted the same compounds in an inverse ratio (4.9% and 13.9%, respectively). Radioactivity appeared in the blood only 24 hr after oral administration. Blood kinetics showed a high elimination half-time (91.8 hr), a distribution volume of 259 mL, and a plasmatic clearance of 1.95 mL/hr. All of the parameters calculated from these experiments suggested a slow metabolism of apigenin, with a slow absorption and a slow elimination phase. Thus, a possible accumulation of this flavonoid in the body can be hypothesized. Metabolism / Metabolites Ether extracts of the urine of male Wistar rats administered apigenin (200 mg) orally contained the phenolic acid metabolites phydroxyphenylpropionic acid, p-hydroxycinnamic acid, and p-hydroxybenzoic acid. Unreacted apigenin, partially characterized apigenin glucuronides, and ethereal sulfates were also identified. With the exception of p-hydroxybenzoic acid and the apigenin conjugates, all of the metabolites detected in the urine after oral administration were also formed in vitro by rat intestinal microorganisms under anaerobic conditions ... In contrast, these metabolites were not detected in SENCAR mice treated topically with apigenin. Furthermore, no evidence of metabolites were observed from the HPLC profiles of epidermal extracts from apigenin-treated mice ... The main in vitro metabolite of apigenin in rat liver Aroclor 1254-induced microsomes has been identified tentatively as the corresponding 3'-hydroxylated compound, luteolin. Apigenin itself is the 3'-hydroxylated metabolite of chrysin ... Apigenin has known human metabolites that include (2S,3S,4S,5R)-3,4,5-Trihydroxy-6-[5-hydroxy-2-(4-hydroxyphenyl)-4-oxochromen-7-yl]oxyoxane-2-carboxylic acid. Biological Half-Life ... The half-life for apigenin was calculated to be on the order of 12 hr ... |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Two different plant species with similar effects are known as chamomile: German chamomile (Matricaria recutita) and Roman chamomile (Chamaemelum nobile). Both contain similar ingredients, including sesquiterpenes (e.g., bisabolol, farnesene), sesquiterpenelactones (e.g., chamazulene, matricin), flavonoids (e.g., apigenin, luteolin), and volatile oils. Chamomile is used orally as a sedative and for gastrointestinal conditions; it is used topically for wound healing. Both herbal and homeopathic preparations have been used to treat mastitis and cracked, bleeding nipples. Chamomile has been used as a galactogogue; however, no scientifically valid clinical trials support this use. Galactogogues should never replace evaluation and counseling on modifiable factors that affect milk production. Chamomile is "generally recognized as safe" (GRAS) for use in food by the U.S. Food and Drug Administration as a spice, seasoning, or flavoring agent. No data exist on the safety of chamomile in nursing mothers or infants, although rare sensitization may occur (see below). It has been safely and effectively used alone and with other herbs in infants for the treatment of colic, diarrhea, and other conditions, so the smaller amounts expected (but not demonstrated) in breastmilk are likely not to be harmful with usual maternal doses. Note that Clostridium botulinum (botulism) spores have been found in some loose-leaf chamomile teas sold in health food stores. Topical chamomile is a known sensitizing agent, even with homeopathic products. Two women developed contact dermatitis of the nipples and areolas after applying Kamillosan ointment for cracked nipples. The product was purchased in England and contained 10.5% Roman chamomile extracts and oil. Reactions were confirmed to be caused by Roman chamomile by patch testing in both women. Drinking chamomile tea can exacerbate topical skin rashes and has caused anaphylaxis in sensitized individuals. Chamomile has possible cross-reactivity with other members of the aster family (e.g., echinacea, feverfew, and milk thistle). Dietary supplements do not require extensive pre-marketing approval from the U.S. Food and Drug Administration. Manufacturers are responsible to ensure the safety, but do not need to prove the safety and effectiveness of dietary supplements before they are marketed. Dietary supplements may contain multiple ingredients, and differences are often found between labeled and actual ingredients or their amounts. A manufacturer may contract with an independent organization to verify the quality of a product or its ingredients, but that does not certify the safety or effectiveness of a product. Because of the above issues, clinical testing results on one product may not be applicable to other products. More detailed information about dietary supplements is available elsewhere on the LactMed Web site. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk A mother nursing her 3-month-old infant began drinking 1.5 to 2 L daily of a chamomile infusion made by pouring 1.5 L of hot water over 1 to 3 grams of chamomile flowers. Each time after drinking the infusion, she noticed fullness and tenderness of the breasts 4 to 6 hours later. She also found that she was able to pump 90 mL of milk after chamomile use, compared to 60 mL without chamomile use. During this time she was also mildly hypothyroid. |
||
| 参考文献 |
|
||
| 其他信息 |
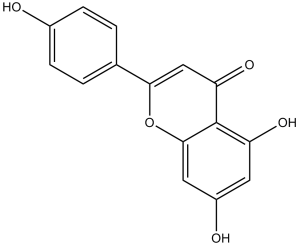
Apigenin is a trihydroxyflavone that is flavone substituted by hydroxy groups at positions 4', 5 and 7. It induces autophagy in leukaemia cells. It has a role as a metabolite and an antineoplastic agent. It is a conjugate acid of an apigenin-7-olate.
Apigenin has been reported in Camellia sinensis, Apis, and other organisms with data available. Apigenin is a plant-derived flavonoid that has significant promise as a skin cancer chemopreventive agent. Apigenin inhibits the expression of involucrin (hINV), a marker of keratinocyte differentiation, is increased by differentiating agents via a protein kinase Cdelta (PKCdelta), Ras, MEKK1, MEK3 cascade that increases AP1 factor level and AP1 factor binding to DNA elements in the hINV promoter. Apigenin suppresses the 12-O-tetradeconylphorbol-13-acetate-dependent increase in AP1 factor expression and binding to the hINV promoter and the increase in hINV promoter activity. Apigenin also inhibits the increase in promoter activity observed following overexpression of PKCdelta, constitutively active Ras, or MEKK1. The suppression of PKCdelta activity is associated with reduced phosphorylation of PKCdelta-Y311. Activation of hINV promoter activity by the green tea polyphenol, (-)-epigellocathecin-3-gallate, is also inhibited by apigenin, suggesting that the two chemopreventive agents can produce opposing actions in keratinocytes. (A7924). Apigenin, a flavone abundantly found in fruits and vegetables, exhibits antiproliferative, anti-inflammatory, and antimetastatic activities through poorly defined mechanisms. This flavonoid provides selective activity to promote caspase-dependent-apoptosis of leukemia cells and uncover an essential role of PKCdelta during the induction of apoptosis by apigenin. (A7925). Apigenin markedly induces the expression of death receptor 5 (DR5) and synergistically acts with exogenous soluble recombinant human tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) to induce apoptosis in malignant tumor cells. On the other hand, apigenin-mediated induction of DR5 expression is not observed in normal human peripheral blood mononuclear cells. Moreover, apigenin does not sensitize normal human peripheral blood mononuclear cells to TRAIL-induced apoptosis. (A7926). 5,7,4'-trihydroxy-flavone, one of the FLAVONES. See also: Flavone (subclass of); Chamomile (part of); Fenugreek seed (part of) ... View More ... Mechanism of Action The dietary flavonoid apigenin (Api) has been demonstrated to exert multiple beneficial effects upon the vascular endothelium. The aim of this study was to examine whether Ca(2+)-activated K(+) channels (K(Ca)) are involved in endothelial nitric oxide (NO) production and antiangiogenic effects ... Endothelial NO generation was monitored using a cyclic guanosine monophosphate radioimmunoassay. K(Ca) activity and changes of the intracellular Ca(2+) concentration [Ca(2+)](i) were analyzed using the fluorescent dyes bis-barbituric acid oxonol, potassium-binding benzofuran isophthalate, and fluo-3. The endothelial angiogenic parameters measured were cell proliferation, [(3)H]-thymidine incorporation, and cell migration (scratch assay). Akt phosphorylation was examined using immunohistochemistry ... Api caused a concentration-dependent increase in cyclic guanosine monophosphate levels, with a maximum effect at a concentration of 1 uM. Api-induced hyperpolarization was blocked by the small and large conductance K(Ca) inhibitors apamin and iberiotoxin, respectively. Furthermore, apamin and iberiotoxin blocked the late, long-lasting plateau phase of the Api-induced biphasic increase of [Ca(2+)](i). Inhibition of Ca(2+) signaling and the K(Ca) blockade both blocked NO production. Prevention of all three (NO, Ca(2+), and K(Ca) signaling) reversed the antiangiogenic effects of Api under both basal and basic fibroblast growth factor-induced culture conditions. Basic fibroblast growth factor-induced Akt phosphorylation was also reduced by Api ... Based on ... /the/ experimental results ... /the authors/ propose the following signaling cascade for the effects of Api on endothelial cell signaling. Api activates small and large conductance K(Ca), leading to a hyperpolarization that is followed by a Ca(2+) influx. The increase of [Ca(2+)](i) is responsible for an increased NO production that mediates the antiangiogenic effects of Api via Akt dephosphorylation. ... Apigenin inhibits the production of proinflammatory cytokines IL-1beta, IL-8, and TNF in LPS-stimulated human monocytes and mouse macrophages. The inhibitory effect on proinflammatory cytokine production persists even when apigenin is administered after LPS stimulation. Transient transfection experiments using NF-kappaB reporter constructs indicated that apigenin inhibits the transcriptional activity of NF-kappaB in LPS-stimulated mouse macrophages. The classical proteasome-dependent degradation of the NF-kappaB inhibitor IkappaBalpha was observed in apigenin LPS-stimulated human monocytes. Using EMSA ... apigenin does not alter NF-kappaB-DNA binding activity in human monocytes. Instead ... apigenin, as part of a non-canonical pathway, regulates NF-kappaB activity through hypophosphorylation of Ser536 in the p65 subunit and the inactivation of the IKK complex stimulated by LPS. The decreased phosphorylation on Ser536 observed in LPS-stimulated mouse macrophages treated with apigenin was overcome by the over-expression of IKKbeta. In addition ... /the/ studies indicate that apigenin inhibits in vivo LPS-induced TNF and the mortality induced by lethal doses of LPS. Collectively, these findings suggest a molecular mechanism by which apigenin suppresses inflammation and modulates the immune response in vivo. Treatment of /human prostate cancer/ LNCaP and PC-3 cells with apigenin causes G0-G1 phase arrest, decrease in total Rb protein and its phosphorylation at Ser780 and Ser807/811 in dose- and time-dependent fashion. Apigenin treatment caused increased phosphorylation of ERK1/2 and JNK1/2 and this sustained activation resulted in decreased ELK-1 phosphorylation and c-FOS expression thereby inhibiting cell survival. Use of kinase inhibitors induced ERK1/2 phosphorylation, albeit at different levels, and did not contribute to cell cycle arrest in comparison to apigenin treatment. Despite activation of MAPK pathway, apigenin caused a significant decrease in cyclin D1 expression that occurred simultaneously with the loss of Rb phosphorylation and inhibition of cell cycle progression. The reduced expression of cyclin D1 protein correlated with decrease in expression and phosphorylation of p38 and PI3K-Akt, which are regulators of cyclin D1 protein. Interestingly, apigenin caused a marked reduction in cyclin D1, D2 and E and their regulatory partners CDK 2, 4 and 6, operative in G0-G1 phase of the cell cycle. This was accompanied by a loss of RNA polymerase II phosphorylation, suggesting the effectiveness of apigenin in inhibiting transcription of these proteins. This study provides an insight into the molecular mechanism of apigenin in modulating various tyrosine kinases and perturbs cell cycle progression, suggesting its future development and use as anticancer agent in humans. The aim of this study was to clarify the anti-inflammatory mechanism of apigenin. Apigenin inhibited the collagenase activity involved in rheumatoid arthritis (RA) and suppressed lipopolysaccharide (LPS)-induced nitric oxide (NO) production in a dose dependent manner in RAW 264.7 macrophage cells. Pretreatment with apigenin also attenuated LPS-induced cyclooxygenase-2 (COX-2) expression. In addition, apigenin profoundly reduced the tumor necrosis factor-alpha (TNF-alpha)-induced adhesion of monocytes to HUVEC monolayer. Apigenin significantly suppressed the TNF-alpha-stimulated upregulation of vascular cellular adhesion molecule-1 (VCAM-1)-, intracellular adhesion molecule-1 (ICAM-1)-, and E-selectin-mRNA to the basal levels. Taken together, these results suggest that apigenin has significant anti-inflammatory activity that involves blocking NO-mediated COX-2 expression and monocyte adherence ... For more Mechanism of Action (Complete) data for APIGENIN (16 total), please visit the HSDB record page. |
| 分子式 |
C15H10O5
|
|
|---|---|---|
| 分子量 |
270.24
|
|
| 精确质量 |
270.052
|
|
| 元素分析 |
C, 66.67; H, 3.73; O, 29.60
|
|
| CAS号 |
520-36-5
|
|
| 相关CAS号 |
Apigenin 7-glucoside;578-74-5
|
|
| PubChem CID |
5280443
|
|
| 外观&性状 |
Light yellow to green yellow solid powder
|
|
| 密度 |
1.5±0.1 g/cm3
|
|
| 沸点 |
555.5±50.0 °C at 760 mmHg
|
|
| 熔点 |
>300 °C(lit.)
|
|
| 闪点 |
217.1±23.6 °C
|
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
|
| 折射率 |
1.732
|
|
| LogP |
2.1
|
|
| tPSA |
90.9
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
1
|
|
| 重原子数目 |
20
|
|
| 分子复杂度/Complexity |
411
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
KZNIFHPLKGYRTM-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H
|
|
| 化学名 |
5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (7.70 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (7.70 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: 10 mg/mL (37.00 mM) in 0.5% CMC-Na/saline water (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7004 mL | 18.5021 mL | 37.0041 mL | |
| 5 mM | 0.7401 mL | 3.7004 mL | 7.4008 mL | |
| 10 mM | 0.3700 mL | 1.8502 mL | 3.7004 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05999682 | Completed | Other: apigenin Other: sterilized water |
Sepsis Septic Shock |
Zhujiang Hospital | September 1, 2023 | Phase 1 Phase 2 |
| NCT03526081 | Completed | Other: Chamomile Tea Other: Parsley based drink |
Healthy | University of California, Davis | January 20, 2015 | Not Applicable |
| NCT03139227 | Withdrawn | Procedure: Bio specimen Collection Dietary Supplement: Dietary Intervention |
Health Status Unknown | Ohio State University Comprehensive Cancer Center |
August 15, 2017 | Not Applicable |
| NCT05788705 | Not yet recruiting | Dietary Supplement: "apigenin" and "glycyrrhizin" |
Rheumatoid Arthritis | Adel A.Gomaa | July 2023 | Not Applicable |
|
|---|
|
|