| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
| 靶点 |
KRAS(G12C)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:ARS-1620 是一种新型、强效、口服的 KRASG12C 共价抑制剂,对 KRASG12C 具有高效力和选择性。它是通过基于结构的设计确定的,可以实现快速、持续的体内靶点占据,从而诱导肿瘤消退。最近发现 KRASG12C 通过等位基因特异性共价靶向诱导型变构开关 II 口袋 (S-IIP) 附近的 Cys-12 具有潜在的药物作用。这种方法的成功需要 KRASG12C 在其活性 GTP 和非活性 GDP 构象之间主动循环,因为 S-IIP 的可及性仅限于 GDP 结合状态。事实证明,该策略对于体外抑制突变型 KRAS 是可行的;然而,尚不确定这种方法是否会转化为体内。 ARS-1620 实现快速、持续的体内靶点占据,诱导肿瘤消退。这项研究提供了体内证据,证明突变型 KRAS 可以被选择性靶向,并揭示 ARS-1620 代表新一代 KRASG12C 特异性抑制剂,具有良好的治疗潜力。激酶测定:ARS-1620 在 p.G12C 突变癌细胞中以高效力和阻转异构体选择性共价抑制 KRAS (G12C) 活性。 ARS-1620 以浓度和时间依赖性方式快速与 G12C 结合,与其共价抑制机制一致。在一组含有突变等位基因的细胞系中,ARS-1620 在处理 2 小时后,在约 0.3 μM 时表现出半最大 G12C 靶点接合 (TE50),在 3.0 μM 时表现出接近完全接合。 RS-1620 在 H358 (p.G12C) 中以剂量依赖性和选择性的方式抑制 RAS-GTP 以及 MEK、ERK、RSK、S6 和 AKT 的磷酸化,但在缺乏 p.G12C 的阴性对照肺癌细胞系中则不然。 A549、H460 和 H441)。 ARS-1620 引发亚微摩尔等位基因特异性效力(IC50 = 0.3 μM;IC90 = 1 μM)。 ARS-1620 的活性对 G12C 等位基因具有特异性,并由 Cys-12 的共价修饰介导。细胞测定:将细胞接种到 24 孔 ULA 板中并静置过夜。然后用 DMSO 或 ARS-1620 处理细胞。处理 2 天后,通过使用annexinV-APC 和碘化丙啶染色或通过 70% 乙醇固定,然后进行 FxCycle 紫染色来测量细胞凋亡和细胞死亡,以通过流式细胞术测量 DNA 含量(细胞周期)和亚二倍体事件的百分比
|
| 体内研究 (In Vivo) |
单次口服剂量或连续 5 次每日剂量后,ARS-1620 产生的平均肿瘤峰值浓度分别为 1.5 μM (50 mg/kg) 和 5.5 μM (200 mg/kg),从而实现显着的 KRASG12C 靶点占有率 (>=70 % G12C-TE (200 mg/kg) 持续 >24 小时。在 MIAPaCa2 异种移植物 (p.G12C) 中,ARS-1620 以剂量依赖性方式显着抑制肿瘤生长 (p<0.001),每天一次给予 200 mg/kg 剂量时,ARS-1620 显着消退。在所有采用的肿瘤模型中,ARS-1620 在整个 3 周的治疗期内具有良好的耐受性。此外,即使在 7 天的时间内每天口服剂量高达 1,000 mg/kg,也没有在 CD-1 小鼠中观察到 ARS-1620 的临床症状或毒性。
ARS-1620在PDX肿瘤模型中显示出强效和选择性的抗肿瘤活性[1] 为了进一步证明ARS-1620的治疗效果,我们分析了一组携带KRAS p.G12C(n=4)的患者衍生(PDX)肿瘤异种移植物模型的抗肿瘤反应,并与缺乏突变等位基因(n=3)的PDX模型进行了比较(靶向测序结果另见表S4)。p.G12C PDX小组由三个腺癌NSCLC组成,这是患者KRAS p.G12C突变频率最高的潜在靶点(11%-16%)(Campbell等人,2016,Jordan等人,2017),以及一个胰腺癌,占KRAS突变胰腺癌的一小部分(<2%)(Bailey等人,2016)。 ARS-1620在p.G12C PDX模型中诱导了显著的TGI(p<0.001),并在使用每日200mg/kg方案治疗3周后出现了明显的消退,而非G12C异种移植物则没有任何反应(图7A)。在研究结束时(最后一次给药后6小时),我们使用每日200mg/kg的治疗方案,确认ARS-1620在肿瘤中的G12C靶点占有率平均≥75%(图7B)。此外,在p.G12C PDX模型中,ARS-1620显著抑制了p-ERK和p-S6(p<0.05)(图7C、S6A和S6B)。通过切割的半胱氨酸天冬氨酸蛋白酶-3的免疫组织化学(IHC)染色监测,这导致了显著的凋亡诱导(p=0.0148)(图7D)。我们接下来评估了以更高剂量或频率给药ARS-1620是否可以提高荷瘤动物的靶点占有率和疗效。200 mg/kg每日两次(b.i.d.)或400 mg/kg每日一次的ARS-1620方案在p.G12C PDX模型中均提高了反应率(图7A),并与更强的p-ERK抑制有关(图S6A-S6C),与细胞系衍生的异种移植物模型中观察到的更大的G12C占用率一致(图S5E-S5H)。在所有采用的肿瘤模型中,ARS-1620在整个3周的治疗期内耐受良好(图S6D和S6E)。此外,即使在7天内每天口服高达1000mg/kg的剂量,CD-1小鼠中也没有观察到ARS-1620的临床症状或毒性(见STAR方法中的动物研究)。高于400mg/kg时,ARS-1620表现出PK饱和,因此在细胞系衍生和PDX衍生的模型中没有进一步增强抗肿瘤疗效(数据未显示)。总之,ARS-1620通常耐受良好,小鼠未达到最大耐受剂量(MTD)。总之,ARS-1620在各种KRASG12C小鼠癌症模型中的体内疗效和突变选择性观察结果支持了未来共价靶向KRAS的S-IIP的治疗策略。 |
| 酶活实验 |
在p.G12C突变癌症细胞中,ARS-1620以共价和高效力选择性抑制KRAS(G12C)活性。根据其共价抑制机制,ARS-1620以浓度和时间依赖的方式快速与G12C结合。与携带突变变体的一组细胞系相比,ARS-1620在处理2小时后,在3.0μM时表现出接近完全的结合,在约0.3μM时显示出一半最大的G12C靶标结合(TE50)。在H358(p.G12C)中,但不在缺乏p.G12C的阴性对照癌症细胞系(A549、H460和H441)中,RS-1620选择性地和剂量依赖性地抑制RAS-GTP和MEK、ERK、RSK、S6和AKT的磷酸化。ARS-1620的效力是亚微摩尔等位基因特异性的(IC50=0.3μM;IC90=1μM)。Cys-12被ARS-1620共价修饰以介导其活性,这是G12C等位基因所独有的。
蜂窝KRASG12C目标参与度(G12C-TE)[1] 细胞(30-50x103)按所列时间用指定化合物处理,随后用PBS洗涤两次,并如前所述制备蛋白质提取(Patricelli等人,2016)。在碘乙酰胺烷基化和胰蛋白酶消化后,使用Skyline targeted Mass Spec Environment v3.6软件(MacLean等人,2010)在Dionex RSLCnano LC上通过靶向LC/MS-MS分析样品,并结合Q-Exactive四极轨道阱质谱仪,如前所述(Patricelli等人,2016)。动力学G12C目标交战是用KinTek Global Kinetic Explorer建模的(Johnson,2009)。 肿瘤KRASG12C靶点结合(G12C-TE)[1] 使用Precellys珠匀浆器在IP裂解缓冲液中制备肿瘤的蛋白质提取物。等分约400μg蛋白质,并加入1皮摩尔重同位素标记的KRASG12C 1-169 his标记蛋白质(Lys-13C6,15N2和Arg-13C6,15N4)作为内标。重同位素标记的KRASG12C在大肠杆菌中产生,蛋白质纯度高于90%,同位素纯度超过99%。使用丙酮沉淀蛋白质,并将其重新悬浮在LDS样品/还原缓冲液中,使用10%NuPAGE-Bis-Tris凝胶通过SDS-PAGE进行分离,随后用考马斯亮蓝染色。从每条泳道上切下覆盖15-25kDa的凝胶带,然后在凝胶内胰蛋白酶消化凝胶包埋的蛋白质。如前所述(Patricelli等人,2016),使用Skyline targeted mass Spec Environment v3.6软件(MacLean等人,2010)在Dionex RSLCnano LC上结合Q-Exactive四极轨道阱质谱仪进行靶向LC/MS-MS分析,分析凝胶中释放的肽。简而言之,前体反应监测用于定量内源性和重同位素标记的胰蛋白酶KRASG12C肽LVVVGAC∗GVGK和KRAS-NRAS标准化肽SYGIPFIETSAK。根据内源性和重同位素标记蛋白的峰面积计算肽轻/重(L/H)同位素比值。参与百分比根据以下公式确定: 拼音双语对照 |
| 细胞实验 |
在接种到24孔ULA板中后,将5×104细胞静置过夜。之后,将DMSO或ARS-1620应用于细胞。两个治疗日后,使用流式细胞术来评估细胞凋亡和细胞死亡的量,以测量亚二倍体事件的百分比和DNA含量(细胞周期),或通过膜联蛋白V-APC和碘化前啶染色或70%乙醇固定,然后进行FxCycle Violet染色[1]。
细胞增殖试验[1] 为了比较抗生长活性,使用了基于CellTiter Glo(CTG)发光的测定法。将细胞(每孔800-1200个)接种(使用相同的培养基)在标准组织培养处理的96孔格式板或超低附着表面96孔格式的板中。在接种后的第二天,用9点3倍稀释的指定化合物系列(每孔100μl最终体积)处理细胞,5天后根据制造商的建议说明监测细胞存活率,其中加入50μl CellTiter Glo试剂,剧烈混合,覆盖并放置在摇床上20分钟,以确保在评估发光信号之前完全溶解细胞。 对于诱导型KRASG12V拯救实验,在有或没有强力霉素(100 ng/ml)、最终培养基体积为90μl的情况下,将细胞作为3D悬浮液(超低粘附板)接种。24小时后,向培养物中加入化合物或DMSO(10μl)的浓度。5天后使用如上所述的CTG测定法监测剩余细胞数。 对于使用无Ras MEFs的实验,细胞以3D悬浮液(超低粘附板)的形式接种。在接种后的第二天,用9点3倍稀释的指定化合物系列(每孔100μl最终体积)处理细胞,5天后通过CTG测定监测细胞存活率。 细胞周期和凋亡测定[1] 将5x104个细胞接种到24孔ULA板中,并静置过夜。然后用DMSO或指定化合物处理细胞。治疗2天后,通过膜联蛋白V APC和碘化丙啶染色或70%乙醇固定,然后进行FxCycle Violet染色,通过流式细胞术测量DNA含量(细胞周期)和亚二倍体事件的百分比,从而测量凋亡和细胞死亡。在MACSQuant流式细胞仪上采集数据,并用FlowJo软件V.10.1进行分析。 免疫印迹和RAS-GTP下拉[1] 来自活性Ras检测试剂盒的1X裂解缓冲液(25 mM Tris-HCl,pH 7.2,150 mM NaCl,5 mM MgCl2,5%甘油,1%NP40)补充了磷酸酶抑制剂和无EDTA蛋白酶抑制剂(蛋白酶抑制剂鸡尾酒桌),用于细胞裂解。对于评估RAS-GTP的裂解物,我们遵循了制造商推荐的程序。简而言之,用冰冷的PBS冲洗0.5-1x106预先粘附的细胞(24小时前),或者如果在3D球体悬浮液(0.5-1x103)中,细胞(在24小时前接种在超低粘附板中)以300 x g的速度造粒3分钟,并用冰冷的PBS:洗涤。之后,用1ml(或0.5ml)含有80μg/ml GST标记的RAF-RBD的裂解缓冲液在冰上裂解细胞10分钟。刮掉剩余的贴壁细胞,在4°C下以14000 rpm离心裂解物5分钟。随后,在4°C下,在恒定摇动下,将90%的预先清除的裂解物加入预先洗涤的谷胱甘肽琼脂糖珠中1小时。随后将珠粒造粒并洗涤3次,用40-60μl 1X SDS-PAGE样品缓冲液洗脱进行蛋白质印迹。剩余的10%裂解物用于通过Bradford蛋白测定法和蛋白质印迹法测定蛋白质浓度,以确定指示的信号标志物。 对于超过24小时的时间过程实验,对RAS-GTP下拉试验进行了小幅修改,以解释治疗引起的细胞数量和/或凋亡诱导的显著差异。为此,细胞裂解物用半体积裂解缓冲液处理,不添加RBD。保存一小部分裂解物样品用于蛋白质浓度测定,其余裂解物被快速冷冻。确保等量的蛋白质经历RBD下拉;随后将裂解物解冻(在室温下),并用裂解缓冲液(0.5mL体积)调节至1mg/ml。然后将等量的裂解物加入到含有RAF-RBD的0.5mL裂解缓冲液中(总体积1mL)。将裂解物涡旋,在冰上孵育10分钟,随后在4°C下以14000 rpm预清5分钟。其余步骤与上述类似。 半胱氨酸选择性分析[1] H358细胞(5x106)用指定的化合物和浓度处理4小时。随后洗涤细胞并收获,如前所述(Patricelli等人,2016),通过LC/MS-MS进行蛋白质组学分析。简而言之,细胞裂解物中暴露于溶剂的半胱氨酸用100μM碘乙酰胺去硫生物素标记。胰蛋白酶消化后,使用高容量链霉抗生物素蛋白琼脂糖富集去硫生物素化肽。使用与Q-Exactive Plus质谱仪耦合的Dionex RSLCnano-LC分析肽样品。Progenesis LC-MS for proteomics v3.0软件用于整个数据集的自动运行比对、峰提取、归一化和峰丰度计算。使用Proteome Discoverer v1.4通过数据库搜索获得去硫生物素化肽的鉴定。将每种肽的标准化峰丰度导出到Excel 2013。计算每个样品组的平均值和%CV,每个样品组包含3个生物重复。计算DMSO对照组和处理样品组之间的Log2倍变化。对每种肽进行双尾t检验,以确定处理组和DMSO对照组之间的统计学意义,假设方差相等。 |
| 动物实验 |
Male BALB/c mice that are 6 to 8 weeks old are used for pharmacokinetic (PK) studies. Mice are given ARS-1620 by oral gavage administration at a dose of 10 mg/kg or a single intravenous (IV) bolus to assess oral bioavailability. The amount of ARS-1620 in plasma is measured using LC-MS/MS techniques. Mean plasma concentration-time profiles are used to estimate pharmacokinetic parameters. Using the linear trapezoidal rule, the area under the curve (AUC) is computed from time versus concentration data. The ratio of the AUC for ARS-1620 from the oral and IV dosage is used to compute the oral bioavailability. Relative doses are used to normalize the computation[1].
Pharmacokinetic (PK) studies in mice [1] For pharmacokinetic (PK) studies 6- to 8-week-old male BALB/c mice were used. To determine oral bioavailability, mice were treated with ARS-1620 by a single intravenous (IV) bolus or oral gavage administration at the doses of 2 and 10 mg/kg, respectively. ARS-1620 was formulated in water solution with 1% N-methyl-2-pyrrolidone, 19% polyethylene glycol 400, and 10% cyclodextrin and then sterilized by filtration for IV dosing. Oral formulation was prepared in solution (100% Labrasol®, Gattefossé). Drug concentration in plasma was quantified by LC-MS/MS-based methods. Pharmacokinetic parameters were estimated using Phoenix WinNonlin from mean plasma concentration-time profiles. The area under the curve (AUC) was calculated from time versus concentration data using the linear trapezoidal rule. The oral bioavailability is calculated as the ratio of AUC for ARS-1620 from oral and IV dosage. The calculation is normalized by relative doses. In all experiments, ARS-1620 displayed greater than 50% oral bioavailability. For PK analysis from tumor samples, vials containing tumor samples were added with 5-fold water and homogenized with a bead mill homogenizer. For calibration standard or QC samples, 40 μl of blank tumor homogenate was transferred into each well of a 96-well removable tube plate and spiked with 10 μl of 5X standard stock spiking solution of ARS-1620 prepared in 100% DMSO. For PK samples, 40 μl of tumor homogenate was transferred into each well of a 96-well removable tube plate and spiked with 10 μl of 100% DMSO. All samples were added with 150 μl of 100% ice-cold acetonitrile containing internal standard and vortexed to ensure thorough mixing. Samples were centrifuged at 3,400 rpm for 10 min and the clean supernatants (30 μl) were transferred into 96-well plate containing 170 μl water with 0.1% formic acid. The plate was capped and briefly vortexed to ensure thorough mixing of the extracted samples. The samples were subjected to LC-MS/MS analysis using an Agilent Technologies 6430 Triple Quad LC/MS system. A Phenomenex Gemini-NX column (C18, 3 μm, 110 Å, 20 mm x 2.0 mm) was used for the LC-MS/MS analysis with mobile phase A containing 10 mM NH4HCO3 in water (pH 10, adjusted by NH4OH) and mobile phase B containing 100% acetonitrile. The LC gradient started with 10% B at time zero till 0.3 minutes, and then was increased to 90% B at 2 minutes. The gradient was decreased from 90% B to 10% B from 2.4 minutes to 2.5 minutes, and then the column was equilibrated at 10% B till 3 minutes. The mass peak of ARS-1620 was monitored by multiple reaction monitoring (MRM) using transition of 431.1 > 124.1 amu. Chromatogram signals were integrated and calibrated using Agilent MassHunter Workstation Software B.06.00. Pharmacokinetic parameters were derived by non-compartmental analysis using Phoenix WinNonlin (version 6.3) from individual tumor concentration versus time profiles. Results are expressed as mean ± s.d. No further statistical analysis was performed. Toxicity assessment in mice [1] For toxicity assessment 6- to 8- week-old male and female CD-1 mice were administered ARS-1620 via oral gavage at doses of 0, 200, 600, and 1,000 mg/kg per day in 100% Labrasol® at 10ml/kg (n = 12 mice per group). Mortality, clinical observations, body weight, hematology, clinical chemistry, organ weights, and toxicokinetics were evaluated. No test article-related clinical observations occurred during the study. No significant drop in body weight was observed. No adverse hematological findings were found except mild increases in neutrophils at 1,000 mg/kg. No test article-related changes in clinical chemistry were noted. No test article-related changes in gross organ weights were found. Mild macroscopic inflammation in the stomach occurred at 1,000 mg/kg/day. No other indications of systemic toxicity were observed. No test article-related changes in microscopic evaluations of organs other than stomach irritation were found. At 1,000 mg/kg/day, Cmax and AUC0-24hr in males and females at the end of study were 5,000-9,000 ng/ml and 35,000-131,000 ng⋅h/mL, respectively. |
| 药代性质 (ADME/PK) |
Following a single oral dose or 5 consecutive daily doses, ARS-1620 yielded average peak tumor concentrations of 1.5 μM (50 mg/kg) and 5.5 μM (200 mg/kg), respectively, that enabled significant KRASG12C target occupancy (≥70% G12C-TE at 200 mg/kg) for >24 hr (Figures 5B and S5A). At these exposures, ARS-1620 elicited a dose- and time-dependent inhibition of RAS-GTP that tracked with covalent G12C modification in xenografts of MIA-PaCa2 and H358 following a single dose (Figures 5C, S5B, and S5C). The target coverage also extended to a 3-day consecutive daily dose schedule of ARS-1620 (200 mg/kg), providing significant G12C target occupancy (75% to 90% G12C-TE) as well as RAS-GTP and downstream signaling inhibition (Figure 5D).[1]
|
| 参考文献 | |
| 其他信息 |
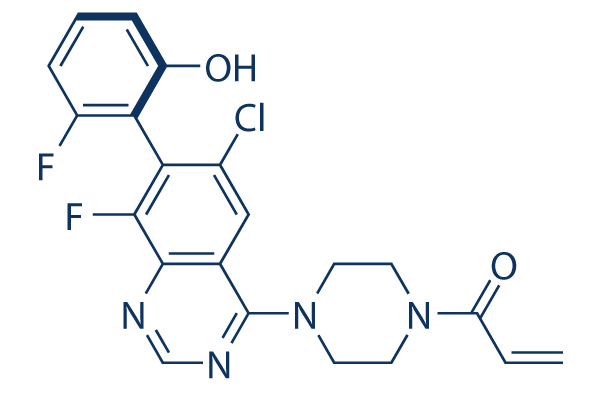
ARS-1620 is a qinazoline derivative carrying chloro and fluoro substituents at positions 6 and 8 respectively, a 2-fluoro-6-hydroxyphenyl group at position 7, and a 4-(prop-2-enoyl)piperazin-1-yl group at position 4. A potent, selective, and orally bioavailable covalent KRAS-G12C inhibitor, it inhibits the protein coding gene KRAS (Kirsten rat sarcoma virus) with high potency in cells and animals. It has a role as an inhibitor, an antiviral agent and an antineoplastic agent.
KRASG12C was recently identified to be potentially druggable by allele-specific covalent targeting of Cys-12 in vicinity to an inducible allosteric switch II pocket (S-IIP). Success of this approach requires active cycling of KRASG12C between its active-GTP and inactive-GDP conformations as accessibility of the S-IIP is restricted only to the GDP-bound state. This strategy proved feasible for inhibiting mutant KRAS in vitro; however, it is uncertain whether this approach would translate to in vivo. Here, we describe structure-based design and identification of ARS-1620, a covalent compound with high potency and selectivity for KRASG12C. ARS-1620 achieves rapid and sustained in vivo target occupancy to induce tumor regression. We use ARS-1620 to dissect oncogenic KRAS dependency and demonstrate that monolayer culture formats significantly underestimate KRAS dependency in vivo. This study provides in vivo evidence that mutant KRAS can be selectively targeted and reveals ARS-1620 as representing a new generation of KRASG12C-specific inhibitors with promising therapeutic potential. [1] The strength and breadth of KRAS dependency across KRAS mutant cancers in vivo has remained underexplored due to the lack of a pharmacological tool. RNAi-based approaches have yielded numerous accounts of variable sensitivity to KRAS silencing using KRAS mutant cancer cell lines and large-scale shRNA screens (Hayes et al., 2016, Lamba et al., 2014, McDonald et al., 2017, Singh et al., 2009, Sunaga et al., 2011, Vartanian et al., 2013). Differential sensitivity to continued expression of mutant KRAS has also been affirmed when examined under adherent monolayer versus 3D culture settings (Fujita-Sato et al., 2015, Patricelli et al., 2016, Vartanian et al., 2013). In this report, we investigated KRAS dependency in the setting of KRAS p.G12C cancer cell lines. Utilizing ARS-1620 as a pharmacologic tool, this study systematically demonstrates the correlation of oncogenic KRAS dependency between in vitro systems and in vivo NSCLC tumor models. We incorporated in our study G12C mutant cancer cells with a range of sensitivity to KRAS depletion confirmed by a meta-analysis from the Project DRIVE sensitivity network (McDonald et al., 2017). Using this cell line panel, we confirmed differential sensitivity to KRAS inhibition in vitro with ARS-1620 in cell lines with both low and high KRAS dependency scores and also confirmed culture-dependent effects of KRAS dependency across monolayer versus 3D-spheroids. We now can extend these KRAS-dependency relationships to the in vivo setting. The finding that ARS-1620 is highly efficacious as a single agent in multiple cell line- and patient-derived mouse xenograft models highlights the central importance of mutant KRAS driving cancer growth and survival in vivo. Moreover, our findings not only imply that 3D-spheroid cultures better predict the in vivo sensitivity of KRAS mutant cancer cells to ARS-1620, they lend support that in vitro studies assessing KRAS dependency using adherent monolayer cell cultures significantly underestimate KRAS dependence in vivo. This has dramatic translational implications for interpreting in vitro synthetic lethal relationships of KRAS as a driving oncogene. Although 3D cultures are becoming more frequently appreciated and utilized to better mimic the in vivo environment and response to chemotherapy (Selby et al., 2017) and other therapeutic targets (e.g., HER2 and EGFR) (Ekert et al., 2014, Howes et al., 2014, Pickl and Ries, 2009, Weigelt et al., 2010), we are not aware of any approved oncology drugs that display differential activity between 2D and 3D cultures as substantial as KRAS inhibition. The patient response rate in future clinical trials to a KRASG12C-directed drug will be an excellent test of the value of using 3D cultures to predict clinical response to therapeutics. Collectively, the in vivo evidence that ARS-1620 is broadly efficacious as a single agent across NSCLC models provides proof of concept that a significant portion of patients with p.G12C KRAS mutations may benefit from KRASG12C-directed therapies. Our study provides the first in vivo evidence that the S-IIP targeted approach may be a promising therapeutic strategy for patients with KRAS p.G12C mutant cancers.[1] |
| 分子式 |
C21H17CLF2N4O2
|
|
|---|---|---|
| 分子量 |
430.8351
|
|
| 精确质量 |
430.1
|
|
| 元素分析 |
C, 58.54; H, 3.98; Cl, 8.23; F, 8.82; N, 13.00; O, 7.43
|
|
| CAS号 |
1698055-85-4
|
|
| 相关CAS号 |
ARS-1323; 1698024-73-5; ARS-1630; 1698055-86-5
|
|
| PubChem CID |
137003167
|
|
| 外观&性状 |
Off-white to light yellow solid powder
|
|
| LogP |
4
|
|
| tPSA |
69.6Ų
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
30
|
|
| 分子复杂度/Complexity |
636
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
ClC1=C(C2C(=CC=CC=2O)F)C(=C2C(=C1)C(=NC=N2)N1CCN(C(C=C)=O)CC1)F
|
|
| InChi Key |
ZRPZPNYZFSJUPA-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C21H17ClF2N4O2/c1-2-16(30)27-6-8-28(9-7-27)21-12-10-13(22)17(19(24)20(12)25-11-26-21)18-14(23)4-3-5-15(18)29/h2-5,10-11,29H,1,6-9H2
|
|
| 化学名 |
1-[4-[6-chloro-8-fluoro-7-(2-fluoro-6-hydroxyphenyl)quinazolin-4-yl]piperazin-1-yl]prop-2-en-1-one
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.83 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.83 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.83 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 5%DMSO + 40%PEG300 + 5%Tween 80 + 50%ddH2O: 4.3mg/ml (9.98mM) 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3210 mL | 11.6052 mL | 23.2105 mL | |
| 5 mM | 0.4642 mL | 2.3210 mL | 4.6421 mL | |
| 10 mM | 0.2321 mL | 1.1605 mL | 2.3210 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。