| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 靶点 |
Anti-malarial; STAT-3; exported protein 1 (EXP1).
|
|---|---|
| 体外研究 (In Vitro) |
青蒿琥酯抑制输出蛋白 1 (EXP1)[2] 和 STAT-3[1]。青蒿琥酯治疗 24 小时后,两种细胞系的活性氧 (ROS) 均出现显着的剂量依赖性升高。此外,Western blotting 证明,以更大剂量的青蒿琥酯处理癌细胞 24 小时可显着提高 γ-H2AX 水平。此外,在 A2780 和 HO8910 细胞中,青蒿琥酯对 RAD51 水平表现出时间依赖性影响。两种类型的非恶性细胞(正常人成纤维细胞和永生化上皮细胞 FTE-187)在响应青蒿琥酯时未显示 RAD51 水平发生变化。事实上,青蒿琥酯以剂量依赖性方式降低 A2780 细胞中的 RAD51 mRNA 水平。相应地,青蒿琥酯显着降低了RAD51的启动子活性。相反,青蒿琥酯对H8910细胞中RAD51 mRNA的量没有影响[3]。
对STL候选物的虚拟分析表明,青蒿琥酯(ATS)是STAT-3的最佳潜在抑制剂,其效力与特异性抑制剂S3I-201相当。我们还观察到,在无细胞系统中,ATS抑制IL-6驱动的STAT-3-DNA结合活性,其效力与S3I-201相当。此外,观察到ATS在体外干扰STAT-3二聚化和抑制组成型和IL-6诱导型STAT-3。然而,我们还观察到,ATS调节STAT-3依赖性靶点(前天冬氨酸蛋白酶-3、Bcl-xl和存活素)有利于体外凋亡的发生。总体而言,ATS对STAT-3的推定抑制表明其通过与STAT-3单体的SH2结构域结合来干扰STAT-3二聚化的能力。它导致STAT-3的抑制,也有利于促进体外细胞凋亡。因此,ATS在体外也表现出癌症细胞对正常细胞的选择性细胞毒性。[1]
EXP1能有效降解细胞毒性血红素,被青蒿琥酯有效抑制,并与青蒿琥乙酯代谢和药物压力疟疾寄生虫的易感性有关。这些数据表明EXP1参与了一线抗疟疾药物的作用模式。[2] 青蒿酸盐诱导卵巢癌症细胞ROS和DNA双链断裂。 青蒿酸下调卵巢癌症细胞中的RAD51。 青蒿琥酯抑制RAD51病灶和HRR的形成。 青蒿酸盐使卵巢癌症细胞对顺铂敏感。 RAD51的异位表达减弱了青蒿琥酯增加的化疗敏感性。 [3] 青蒿琥酯在体外具有强大的抗淋巴瘤活性[4] 为了表征青蒿琥酯的疗效,在药物敏感性筛查中,将目前临床试验中用于治疗B细胞淋巴瘤的抑制剂与青蒿琥乙酯进行了比较。青蒿琥酯被确定为一种有前景的候选药物,因为它在所有测试的B淋巴瘤细胞系中都能有效减少细胞生长,这与其他药物的疗效存在较大差异(图1)。为了扩大青蒿琥酯的测试范围,在代表各种组织学类型的18种不同B细胞淋巴瘤细胞系中进行了剂量反应实验。这表明青蒿琥酯在淋巴瘤细胞系中表现出广泛的活性,并强烈影响细胞生长,而在单独用CD40L或与IL21联合激活的正常B细胞中,其作用有限(图2a)。青蒿琥酯降低了细胞生长,IC50值从0.189μM降至3.72μM,在测试的18个细胞系中,有11个对青蒿琥乙酯高度敏感(IC50<1μM)(图2a,附加文件2:图S1)。72小时后,PI染色检测到明显的细胞死亡,对正常B细胞只有轻微影响(附加文件2:图S1B)。TUNEL法检测DNA断裂表明,青蒿琥酯诱导的凋亡很强,暴露于青蒿琥乙酯72小时后,凋亡细胞百分比为55至96,而正常B细胞不受影响(图2b)。凋亡的诱导是一个早期事件,青蒿琥酯处理24小时后,活性胱天蛋白酶3+细胞显著增加(附加文件2:图S1C)。青蒿琥酯对活性胱天蛋白酶3+细胞的增加被泛胱天蛋白酶抑制剂Z-VAD-FMK的存在所抵消(图2c),表明青蒿琥乙酯通过胱天蛋白酶依赖途径诱导肿瘤细胞凋亡。细胞周期分析没有显示青蒿琥酯引起的任何总体变化(附加文件3:图S2)。总之,这些结果表明,诱导细胞凋亡是青蒿琥酯抗淋巴瘤活性的主要机制。 |
| 体内研究 (In Vivo) |
青蒿琥酯和顺铂联合治疗组肿瘤的发展明显受到抑制(P<0.01)。另一方面,单独使用青蒿琥酯时,两种细胞系的肿瘤异种移植物均未显着生长[3]。
Artesunate联合顺铂抑制卵巢癌症异种移植物肿瘤生长[3] 接下来,我们在体内测试青琥酯和顺铂单独或联合用药对卵巢癌症细胞生长的影响。A2780和HO8910细胞,5×106个细胞/0.2 ml PBS,皮下注射到雌性裸鼠的左腹股沟区。将携带肿瘤的小鼠随机分为4组,每天通过腹腔注射青蒿琥酯(50mg/kg)、顺铂(2mg/kg)或两者的组合给药16天。如图6所示,接受青蒿琥酯和顺铂联合治疗的组肿瘤生长明显减少(P<0.01)。相比之下,单独使用青蒿琥酯对两种细胞系的肿瘤异种移植物的生长没有显著影响。单独使用顺铂似乎仅对HO8910细胞有一定的抑制作用,但对A2780细胞没有。这些结果表明青琥酯可使卵巢癌症细胞在体内对顺铂敏感。 青蒿琥酯在淋巴瘤异种移植物模型中具有强效的抗淋巴瘤活性[4] 为了测试青蒿琥酯作为体内强效抗淋巴瘤药物的疗效,NSG小鼠皮下接种2×106 BL-41-luc淋巴瘤细胞,随后用青蒿琥酸酯(200mg/kg/天,n=10)治疗或不治疗(对照组,n=10 = 10). 具有不同肿瘤负荷的小鼠在各组中均匀分布(附加文件4:图S3A和B),并在接种后第4天开始治疗。青蒿琥酯治疗的小鼠肿瘤生长明显减少,治疗开始后第12天和第16天达到统计学上的显著差异(P = .0045, 第12天,P<0.0050,第16天;图3a)。第16天小鼠的IVIS图像显示,与对照组相比,青蒿琥酯治疗的小鼠肿瘤生长明显减少(图3b)。在一只接受青蒿琥酯治疗的小鼠中,肿瘤无法检测到(图3b)。在第16天,由于最大肿瘤大小限制(2 cm3),一半的对照小鼠被安乐死。相比之下,第一只青蒿琥酯治疗的小鼠在6天后因肿瘤大小而被安乐死。在治疗结束后(第19天)监测所有剩余的小鼠,在青蒿琥酯治疗的小鼠中观察到肿瘤生长延迟。生存分析显示,对照组和青蒿琥酯治疗的小鼠达到最大肿瘤大小标准的中位时间分别为17.5天和30.5天(p < .0001; 图3c)。青蒿琥酯剂量(200mg/kg/天)耐受良好,治疗期间体重没有减轻(附加文件4:图S3C)。总之,青蒿琥酯在侵袭性B细胞淋巴瘤异种移植物模型中显示出强烈的抗肿瘤反应。 |
| 酶活实验 |
EXP1青蒿琥酯抑制试验[2]
将Artesunate/青蒿琥酯(ART)和阿托伐醌溶解在100%乙醇中,加入100 nM WT EXP1血红素反应中,以测定血红素降解抑制作用。将WT EXP1在pH 6.5的GST测定缓冲液中与0.1%Triton X-100和ART在冰上预孵育30分钟,然后加入2mM GSH和血红素。在395nm下每秒监测反应3分钟。 EXP1 GST酶测定[2] 纯化的EXP1在pH 6.5下与0.1%Triton X-100和1mM还原GSH在冰上预孵育90分钟,然后加入CDNB。在340nm下每15秒监测一次反应的吸光度,持续10分钟。通过将100 nM WT蛋白与0.1%Triton X-100、2 mM GSH在pH 6.5的测定缓冲液中在冰上预孵育30分钟,然后加入血红素以启动血红素降解,来测定GST对血红素的活性。在395nm下每秒监测吸光度3分钟。 |
| 细胞实验 |
DHA/ART耐受寄生虫的产生[2]
通过在青蒿素衍生物双氢青蒿素(DHA)存在下以亚IC50浓度(2.8 nM,而亲本IC50值为6.4 nM)培养无性血液期寄生虫(平均约108)55天,然后在接下来的200天内逐渐增加药物浓度(高达28 nM),从Dd2菌株中选择3b1寄生虫。在寄生虫暴露于高浓度DHA/ART四天后(高达112 nM;Eastman等人,手稿正在准备中),对DHA和青蒿琥酯(ART)的获得性耐受被证明在30天内复发频率增加。 ROS的流式细胞术分析[3] 通过膜渗透性CM-H2DCFDA测定青蒿琥酯处理后ROS的产生。简而言之,细胞装载了5μM的CM-H2DCFDA,并在用青蒿琥酯处理后在37°C下孵育20分钟。使用保存液重新悬浮细胞,并用FACSCanto II(Becton Dickinson)进行分析。氧化CM-H2DCFDA的峰值激发波长为490nm,发射波长为530nm。 γ-H2AX和RAD51[3]的免疫荧光染色 在6孔板的盖玻片上生长的细胞用青蒿琥酯处理24小时,然后进行γ-H2AX水平的测定。对于RAD51的免疫荧光染色,细胞用10μg/ml青蒿琥酯预处理24小时,然后用30μM顺铂预处理4小时以诱导RAD51病灶。用4%甲醛固定细胞,然后用0.2%Triton X-100的PBS溶液处理5分钟,然后用5%牛血清白蛋白的PBS溶液封闭30分钟。小鼠抗γ-H2AX抗体在PBS中的5%牛血清白蛋白中以1:600的稀释度使用。兔抗Rad51抗体以1:600稀释。将标本在4°C下孵育过夜。然后在PBS中洗涤细胞三次,然后在黑暗中用罗丹明标记的二抗孵育60分钟。用含有0.3%Triton X-100的PBS洗涤3次后,用4,6-二脒基-2-苯基吲哚复染细胞5分钟。盖玻片用防磨溶液安装在载玻片上。然后在荧光显微镜下检查载玻片。在3次实验重复中,每次至少对500个核进行核灶评分。 蛋白质印迹分析[3] 用青蒿琥酯处理24小时后,收获细胞并在1×细胞裂解缓冲液中裂解。通过SDS-PAGE分离15-25μg的总蛋白质,并将其转移到聚偏二氟乙烯(PVDF)膜上。在室温下用5%脱脂乳封闭膜1-2小时,然后用一抗探测,并在4°C下孵育过夜。用TBS-T广泛洗涤后,将膜与适当的HRP偶联的二抗在室温下孵育1小时,然后用Western ECL增强的鲁米诺反应剂检测。针对以下蛋白质的抗体为RAD51、β-肌动蛋白和His标签。 萤光素酶报告物测定[3] 使用Lipofectamine 2000(Invitrogen)用携带一系列截短RAD51启动子的pGL3碱性报告质粒转染细胞。将细胞与/不与青蒿琥酯(10μg/ml)一起孵育24小时,然后使用带有多标记计数器的双荧光素酶报告检测系统(Victor 1420;PerkinElmer)进行荧光素酶检测。萤火虫荧光素酶活性被标准化为pRL-TK报告物(共转染的内部对照)的肾小管萤光素酶活性。在3个独立实验中进行了转染,并进行了4次检测 克隆存活试验[3] 卵巢细胞分为4组,即Artesunate/青蒿琥酯组(5μg/ml,24小时)、顺铂组(0.3μM,24 h)、联合组(青蒿琥酯和顺铂序贯治疗24小时)和对照组(DMSO)。将细胞接种到6cm培养皿中。10-12天后,用甲醇固定菌落,用1.25%Giemsa染色进行计数。仅对那些大小至少为50个细胞的菌落进行计数。 HR报告分析[3] 含有HR报告盒的质粒由Gorbunova博士提供。13报告盒由2个GFP突变拷贝组成。第一个拷贝包含一个人工内含子和一个22nt的缺失,以及在第一个外显子中反向插入2个I-SceI识别位点。第二个拷贝包含第一个外显子,但缺少启动子和起始密码子。在I-SceI诱导DSB后,基因转换事件重建了一个功能性的GFP基因。质粒通过I-SceI限制性内切酶线性化,并使用天根通用DNA纯化试剂盒纯化。将2μg HR报告构建体和0.1μg pDsRed-N1作为内对照转染指数增长的A2780和HO8910细胞。然后,用青蒿琥酯处理细胞24小时,用新鲜培养基替换,并依次培养48小时。收集细胞,将其重新悬浮在0.4ml pH 7.4的PBS中,并通过FACS进行分析。DNA修复效率以GFP+/DsRed+比值表示,与转染条件无关。 存活率和凋亡测定[4] 细胞在96孔板(10000个细胞/孔)中生长72小时,有或没有青蒿琥酯(0.125-8μM)和小分子抑制剂(附加文件1:表S1)。CellTiterGlo测定用于使用Modulus微孔板读数器测量相对细胞生长(ATP水平)。测量结果与对照组相关,并报告为相对发光单位(RLU)。在用青蒿琥酯(5-10μM)处理72小时后,通过碘化丙啶(PI)测定法(1μg/ml)测量存活率。在用有和没有Z-VAD-FMK(1μl/ml)的青蒿琥酸酯(1和5μM)治疗24小时后,用活性半胱氨酸天冬氨酸蛋白酶-3测定法(Alexa-647-rabbit抗活性半胱氨酸天冬酶-3克隆C92-605)检测细胞凋亡。用青蒿琥酯(10μM)处理72小时后,用原位细胞死亡检测试剂盒测定末端脱氧核苷酸转移酶dUTP缺口末端标记(TUNEL)。对于这两种凋亡测定,细胞在PFA中固定5分钟,并用冰冷的甲醇渗透。所有测定均使用FACS Canto II或LSR II流式细胞仪 进行分析。使用在线Cytobank流式细胞术软件(www.Cytobank.org)分析流式细胞仪数据。DL-丁硫醚磺酰亚胺(BSO)在50μM下使用18-24小时,通过抑制γ-谷氨酰半胱氨酸合成酶来降低细胞谷胱甘肽水平。谷胱甘肽水平通过GSH-Glo谷胱甘肽测定法(Promega)测量。 基因表达谱[4] 在用青蒿琥酯(5μM)处理4小时和12小时后,使用MiRNeasy分离总RNA。微阵列分析在Illumina的HumanHT-12 v4 Expression BeadChip平台上进行。使用R中的limma包(版本3.3.1)进行差异基因表达分析。每个细胞系和时间点进行两次技术复制,并将结果取平均值。基于大于绝对值0.5的对数倍变化和小于0.01的调整后p值(FDR)来选择差异表达的基因。使用Illumina的HumanHT-12 v4 Expression BeadChip平台的注释文件,根据基因符号折叠探针。当多个探针定位到同一基因时,选择对数倍数变化最小的探针。通过默认设置的Ingenuity Pathway Analysis(IPA)软件确定了差异表达基因最丰富的途径和网络。 |
| 动物实验 |
Four to 6 weeks old female athymic nude mice (BALB/c, nu/nu) were purchased from Beijing Experimental Animal Center (Beijing, China). A2780 and HO8910 cells were harvested and resuspended in 0.1 ml of PBS, 5 × 106 cells/0.2 ml were injected subcutaneously into the left inguinal area of the mice. Two weeks later, mice bearing tumors (∼70 mm3 for A2780 and HO8910) were randomly divided into 4 groups. Artesunate was administered daily via i.p. injection at doses of 50 mg/kg alone or in combination with cisplatin (2 mg/kg) for 16 days. The tumor growth was monitored every other day. Tumor volume was determined by the formula 1/2a × b2 where a is the long diameter (mm) and b is the short diameter (mm).
Four to 6 weeks old female athymic nude mice (BALB/c, nu/nu) were used. A2780 and HO8910 cells were harvested and resuspended in 0.1 ml of PBS, 5 × 106 cells/0.2 ml were injected subcutaneously into the left inguinal area of the mice. Two weeks later, mice bearing tumors (∼70 mm3 for A2780 and HO8910) were randomly divided into 4 groups. Artesunate was administered daily via i.p. injection at doses of 50 mg/kg alone or in combination with cisplatin (2 mg/kg) for 16 days. The tumor growth was monitored every other day. Tumor volume was determined by the formula 1/2a × b2 where a is the long diameter (mm) and b is the short diameter (mm).[3] NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were bred in-house. Pilot experiments were performed with three mice in each group. Based on the results, n = 10 for treatment and control group was chosen. The mice (6–10 weeks old) were injected subcutaneously with 2 × 106 BL-41 cells expressing firefly luciferase (BL-41-luc). Tumor take was measured by IVIS at day 4 before mice were divided into control and treatment groups. The mice were divided according to tumor size within each cage for either treatment or control group to have non-biased, comparable groups. Artesunate was dissolved in EtOH/DMSO (1:1) to 370 mg/ml and diluted 1:10 in 5% Na3CO3 before injections. Mice were injected daily from day 4 with 200 mg/kg Artesunate intraperitoneally or control (5% EtOH and 5% DMSO in Na3CO3). Treatment was given for 12 days, then 2 days off, followed by treatment every day until day 19 (17 injections total). Tumor growth was monitored by bioluminescent imaging at regular intervals. Mice were injected intraperitoneally with 150 mg/kg d-luciferin and imaged after 10 min. Inhalation anesthetic sevofluran supplemented with O2 and N2O was used during imaging. Caliper measurement was used to determine if the tumor size had reached maximum 2 cm in one direction or 2 cm3 that was the limit for euthanasia.[4] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The Cmax of artesunate is 3.3µg/mL while the Cmax of the active metabolite DHA is 3.1µg/mL. The AUC of artesunate is 0.7µg\*h/mL while the AUC of DHA is 3.5µg\*h/mL. After intravenous artesunate, DHA has a Tmax of 0.5-15 minutes in adult patients and 21-64 minutes in pediatric patients. Intramuscular artesunate has a Tmax of 8-12 minutes. Infants less than 6 months old will have a higher AUC due to an undeveloped UGT metabolic pathway. The main route of elimination in humans is unknown. In rats, a dose of artesunate is 56.1% eliminated in the urine and 38.5% in the feces. The volume of distribution of artesunate is 68.5L while the volume of distribution of DHA is 59.7L. The clearance of artesunate is 180L/h while the clearance of DHA is 32.3L/h. Following administration to humans, artesunate is rapidly hydrolyzed to its principal active metabolite, dihydroartemisinin. The pharmacokinetics of artesunate are characterized by marked inter-subject variability, differing significantly between healthy volunteers and infected patients, and among patients with different disease severity. The pharmacokinetic of artesunate and dihydroartemisin are characterized by marked inter-subject variability. The pharmacokinetic parameters of artesunate and dihydroartemisinin differ significantly between healthy volunteers and infected patients, and among patients with different disease severity. Pharmacokinetic data from unbound plasma concentrations of artesunate or dihydroartemisinin should be interpreted with caution because the drug accumulates selectively in parasitized RBC's In in vitro experiments, accumulation of dihydroartemisinin in infected RBC's is in concentrations approximately 300-fold higher than those in plasma . The pharmacokinetics of oral dihydroartemisinin (DHA) following the dose of 2 and 4 mg/ kg body weight dihydroartemisinin and 4 mg/kg body weight oral artesunate (AS) were investigated in 20 healthy Thai volunteers (10 males, 10 females). All formulations were generally well tolerated. Oral DHA was rapidly absorbed from gastrointestinal tract with marked inter-individual variation. The pharmacokinetics of DHA following the two dose levels were similar and linearity in its kinetics was observed. Based on the model-independent pharmacokinetic analysis, median (95% CI) values for Cmax of 181 (120-306) and 360 (181-658) ng/ml were achieved at 1.5 hours following 2 and 4 mg/kg body weight dose, respectively. The corresponding values for AUC0-infinity, t1/2z, CL/f and Vz/f were 377 (199-1,128) vs 907 (324-2,289) ng.hr/mL, 0.96 (0.70-1.81) vs 1.2 (0.75-1.44) hours, 7.7 (4.3-12.3) vs 6.6 (3.1-10.1) L/kg, and 90.5 (28.6-178.2) vs 6.6 (3.1-10.1) mL/min/kg, respectively (2 vs 4 mg/kg dose). Oral AS was rapidly biotransformed to DHA, which was detectable in plasma as early as 15 minutes of AS dosing. Following 4 mg/kg dose, median (95% CI) value for Cmax of 519 (236-284) ng/mL was achieved at 0.7 (0.25-1.5) hours. AUC0-infinity, and t1/2z were 657 (362-2,079) ng.hr/mL, 0.74 (0.34-1.42) hours, respectively. Cmax of DHA following oral AS were significantly higher, but total systemic exposure was greater following oral DHA at the same dose level (4 mg/kg body weight). There was no significant sex difference in pharmacokinetics of DHA The aims of this study were to determine the pharmacokinetic parameters of a single dose of 200 mg oral and rectal artesunate in healthy volunteers, and to suggest a rational dosage regimen for rectal administration. The study design was a randomized open cross-over study of 12 healthy volunteers... Pharmacokinetic parameters were derived from the main metabolite alpha-dihydroartemisinin data due to the rapid disappearance of artesunate from the plasma. Dihydroartemisinin following oral administration of artesunate had a significantly higher AUC(0-infinity) (P<0.05 95% confidence interval (CI) -1168.73, -667.61 ng x hr/mL(-1)) and Cmax (P<0.05; 95% CI -419.73, -171.44 ng/mL(-1)), and had shorter tmax (P<0.05; 95% CI -0.97, -0.10 hr) than that following rectal artesunate. There was no statistically significant difference in the elimination half-life between both routes of administration (P>0.05; 95% CI -0.14, 0.53 hr). The relative bioavailability of rectal artesunate was [mean (coefficient of variation %) 54.9 (24.8%) %]. For more Absorption, Distribution and Excretion (Complete) data for ARTESUNIC ACID (8 total), please visit the HSDB record page. Metabolism / Metabolites Artesunate is rapidly metabolized to dihydroartemisinin (DHA) by plasma esterases. DHA is glucuronidated by UGT1A9 and UGT2B7 to DHA-glucuronide. DHA-glucuronide can undergo a minor metabolic pathway to for a furano acetate derivative of DHA-glucuronide. CYP2A6 may minorly contribute to the metabolism of artesunate. Following administration to humans, artesunate is rapidly hydrolyzed to its principle active metabolite, dihydroartemisinin. Data from in vitro studies with human liver microsomes and from clinical studies suggest that DHA-glucoronide (10-position) is the principal Phase II metabolite of DHA and that uridine diphosphate glucuronyl transferase isoforms 1A1, 1A8-9, or 2B7 may be the main conjugating enzyme. Artemisinin is completely and rapidly absorbed after oral administration in rats. However, a very low plasma level was obtained even after a dose of 300 mg/kg. Liver was found to be the chief site of inactivation. When artemisinin was given i.m., significant and more persistent plasma levels were detected. Artemisinin was shown to pass the blood-brain and blood-placenta barriers after i.v. injection. Very little unchanged artemisinin was found in the urine or feces in 48 hours regardless of the route of administration. Metabolites identified after administration to humans include deoxyartemisinin, deoxydihydroartemisinin, and 9,10-dihydroxydeoxyartemisinin. Artesunate has known human metabolites that include (1S,4S,5R,8S,9R,10R,12R,13R)-1,5,9-Trimethyl-11,14,15,16-tetraoxatetracyclo[10.3.1.04,13.08,13]hexadecan-10-ol. Biological Half-Life The elimination half life of artesunate is 0.3h with a range of 0.1-1.8h. The elimination half life of DHA is 1.3h with a range of 0.9-2.9h. Half life after intramuscular administration is 48 min in children and 41 min in adults. In volunteer studies, artsunate was cleared very rapidly (within minutes) by biotransformation to dihydroartemisinin, which was eliminated by with a half-life of approximately 45 minutes. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In an open label study of severe malaria in 104 patients in the United States, elevations in ALT occurred in 27% of subjects, AST in 49%, and bilirubin in 17%. These abnormalities, however, were attributable to the hemolysis and liver involvement that are common in patients with acute, severe malaria. None of the elevations were attributed to drug induced liver injury. Since licensure of artesunate for injection and its more widescale clinical use in the United States, there have been no reports of acute liver injury attributed to its use. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited information indicates that a maternal dose of 200 mg orally produced low levels in milk and would not be expected to cause any adverse effects in breastfed infants, especially if the infant is older than 2 months. Withholding breastfeeding for 6 hours after a dose should markedly reduce the dose the infant receives. In general, very small amounts of antimalarial drugs are excreted in the breast milk of lactating women. Because the quantity of antimalarial drugs transferred in breast milk is insufficient to provide adequate protection against malaria, infants who require chemoprophylaxis must receive the recommended dosages of antimalarial drugs. ◉ Effects in Breastfed Infants Breastfed infants who were given dihydroartemisinin and piperaquine as a treatment for malaria had a higher frequency of vomiting than non-breastfed infants given the drugs. Whether this finding applies to infants who receive dihydroartemisinin via breastmilk has not been studied. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Artesunate and its metabolite DHA are approximately 93% protein bound in plasma. Artesunate can bind to serum albumin. Interactions The activity of artemisinin in combination with other antimalarial drugs against P. falciparum was measured in vitro and against P. berghei in vivo. A combination of artemisinin with mefloquine was synergistic whereas that with pyrimethamine was antagonistic in vitro and in vivo. A combination of artemisinin with other antimalarials (sulfadiazine, sulfadoxine, sulfadoxine-pyrimethamine, cycloguanil, and dapsone) was also shown to be antagonistic in vivo. There has been some concern that antipyretics might attenuate the host defense against malaria, as their use is associated with delayed parasite clearance. However, this appears to result from delaying cytoadherence, which is likely to be beneficial. There is no reason to withhold antipyretics in malaria. ...Paracetamol (acetaminophen) and ibuprofen are the preferred options for reducing fever. |
| 参考文献 |
[1]. Artesunate as an Anti-Cancer Agent Targets Stat-3 and Favorably Suppresses Hepatocellular Carcinoma. Curr Top Med Chem. 2016;16(22):2453-63.
[2]. Supergenomic network compression and the discovery of EXP1 as a glutathione transferase inhibited by artesunate. Cell. 2014 Aug 14;158(4):916-928. [3]. Artesunate sensitizes ovarian cancer cells to cisplatin by downregulating RAD51. Cancer Biol Ther. 2015;16(10):1548-56. [4]. Artesunate shows potent anti-tumor activity in B-cell lymphoma. J Hematol Oncol. 2018 Feb 20;11:23. |
| 其他信息 |
Therapeutic Uses
Therap Cat: Antimalarial Artesunate Rectal Capsules is indicated for the initial management of acute malaria in patients who cannot take medication by mouth and for whom parenteral treatment is not available. To counter the threat of resistance of P. falciparum to monotherapies, and to improve treatment outcome, combinations of antimalarials are now recommended by WHO for the treatment of falciparum malaria. The following ACTs are currently recommended (alphabetical order): AS+AQ artesunate + amodiaquine combination, AS+MQ artesunate + mefloquine combination, AS+SP artesunate + sulfadoxine-pyrimethamine combination. Artemisinin and its derivatives (artesunate, artemether, artemotil, dihydroartemisinin) produce rapid clearance of parasitaemia and rapid resolution of symptoms. They reduce parasite numbers by a factor of approximately 10,000 in each asexual cycle, which is more than other current antimalarials (which reduce parasite numbers 100- to 1000-fold per cycle). Artemisinin and its derivatives are eliminated rapidly. When given in combination with rapidly eliminated compounds (tetracyclines, clindamycin), a 7-day course of treatment with an artemisinin compound is required; but when given in combination with slowly eliminated antimalarials, shorter courses of treatment (3 days) are effective. The evidence of their superiority in comparison to monotherapies has been clearly documented. For more Therapeutic Uses (Complete) data for ARTESUNIC ACID (14 total), please visit the HSDB record page. Drug Warnings Artemisinin congeners should not be given to patients with a previous history of an allergic reaction following their consumption or if an urticarial rash develops during treatment. Patient with a history of hypersensitivity reaction to one of the artemsinins should be advised not to take any of the derivatives again. Artesunate rectal capsules have not been evaluated as sole therapy for malaria; consequently all patient who are initially treated with artesunate rectal capsules should be promptly referred and evaluated at the nearest health care facility able to provide a full curative course of treatment for malaria. Adverse events described /following artesunate/ included bitter taste, mild pain at the injection site, bradycardia, paroxysmal ventricular premature beat, incomplete right bundle branch block, first-degree atrio-ventricular block, and urticaria. ... The most commonly reported adverse events (in the order of <1%) to be mild gastrointestinal (nausea, vomiting, diarrhea, abdominal pain) events. For more Drug Warnings (Complete) data for ARTESUNIC ACID (25 total), please visit the HSDB record page. Pharmacodynamics Artesunate is an artemisinin derivative that is metabolized to DHA, which generates free radicals to inhibit normal function of _Plasmodium_ parasites. It has a short duration of action due to its short half life, and a moderate therapeutic index. Patients should be counselled regarding the risk of post treatment hemolytic anemia and hypersenstivity. A central problem in biology is to identify gene function. One approach is to infer function in large supergenomic networks of interactions and ancestral relationships among genes; however, their analysis can be computationally prohibitive. We show here that these biological networks are compressible. They can be shrunk dramatically by eliminating redundant evolutionary relationships, and this process is efficient because in these networks the number of compressible elements rises linearly rather than exponentially as in other complex networks. Compression enables global network analysis to computationally harness hundreds of interconnected genomes and to produce functional predictions. As a demonstration, we show that the essential, but functionally uncharacterized Plasmodium falciparum antigen EXP1 is a membrane glutathione S-transferase. EXP1 efficiently degrades cytotoxic hematin, is potently inhibited by artesunate, and is associated with artesunate metabolism and susceptibility in drug-pressured malaria parasites. These data implicate EXP1 in the mode of action of a frontline antimalarial drug.[2] Artesunate, a semi-synthetic derivative of arteminisin originally developed for the treatment of malaria, has recently been shown to possess antitumor properties. One of the cytotoxic effects of artesunate on cancer cells is mediated by induction of oxidative stress and DNA double-strand breaks (DSBs). We report here that in addition to inducing oxidative stress and DSBs, artesunate can also downregulate RAD51 and impair DSB repair in ovarian cancer cells. We observed that the formation of RAD51 foci and homologous recombination repair (HRR) were significantly reduced in artesunate-treated cells. As a consequence, artesunate and cisplatin synergistically induced DSBs and inhibited the clonogenic formation of ovarian cancer cells. Ectopic expression of RAD51 was able to rescue the increased chemosensitivity conferred by artesunate, confirming that the chemosensitizing effect of artesuante is at least partially mediated by the downregulation of RAD51. Our results indicated that artesunatecan compromise the repair of DSBs in ovarian cancer cells, and thus could be employed as a sensitizing agent in chemotherapy.[3] Although chemo-immunotherapy has led to an improved overall survival for most B-cell lymphoma types, relapsed and refractory disease remains a challenge. The malaria drug artesunate has previously been identified as a growth suppressor in some cancer types and was tested as a new treatment option in B-cell lymphoma. Methods: We included artesunate in a cancer sensitivity drug screen in B lymphoma cell lines. The preclinical properties of artesunate was tested as single agent in vitro in 18 B-cell lymphoma cell lines representing different histologies and in vivo in an aggressive B-cell lymphoma xenograft model, using NSG mice. Artesunate-treated B lymphoma cell lines were analyzed by functional assays, gene expression profiling, and protein expression to identify the mechanism of action. Results: Drug screening identified artesunate as a highly potent anti-lymphoma drug. Artesunate induced potent growth suppression in most B lymphoma cells with an IC50 comparable to concentrations measured in serum from artesunate-treated malaria patients, while leaving normal B-cells unaffected. Artesunate markedly inhibited highly aggressive tumor growth in a xenograft model. Gene expression analysis identified endoplasmic reticulum (ER) stress and the unfolded protein response as the most affected pathways and artesunate-induced expression of the ER stress markers ATF-4 and DDIT3 was specifically upregulated in malignant B-cells, but not in normal B-cells. In addition, artesunate significantly suppressed the overall cell metabolism, affecting both respiration and glycolysis. Conclusions: Artesunate demonstrated potent apoptosis-inducing effects across a broad range of B-cell lymphoma cell lines in vitro, and a prominent anti-lymphoma activity in vivo, suggesting it to be a relevant drug for treatment of B-cell lymphoma.[4] |
| 分子式 |
C19H28O8
|
|
|---|---|---|
| 分子量 |
384.42
|
|
| 精确质量 |
384.178
|
|
| 元素分析 |
C, 59.36; H, 7.34; O, 33.29
|
|
| CAS号 |
88495-63-0
|
|
| 相关CAS号 |
Artesunate-d3;1316303-44-2;Artesunate-d4;1316753-15-7; 82864-68-4 (Sodium salt)
|
|
| PubChem CID |
6917864
|
|
| 外观&性状 |
Fine white crystalline powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
507.1±50.0 °C at 760 mmHg
|
|
| 熔点 |
132-135ºC
|
|
| 闪点 |
175.6±23.6 °C
|
|
| 蒸汽压 |
0.0±2.8 mmHg at 25°C
|
|
| 折射率 |
1.544
|
|
| LogP |
2.94
|
|
| tPSA |
100.52
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
8
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
27
|
|
| 分子复杂度/Complexity |
623
|
|
| 定义原子立体中心数目 |
8
|
|
| SMILES |
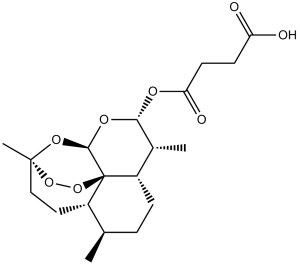
C[C@H]1[C@H](OC(=O)CCC(=O)O)O[C@@H]2O[C@]3(CC[C@@H]4[C@@]2(OO3)[C@H]1CC[C@H]4C)C
|
|
| InChi Key |
FIHJKUPKCHIPAT-AHIGJZGOSA-N
|
|
| InChi Code |
InChI=1S/C19H28O8/c1-10-4-5-13-11(2)16(23-15(22)7-6-14(20)21)24-17-19(13)12(10)8-9-18(3,25-17)26-27-19/h10-13,16-17H,4-9H2,1-3H3,(H,20,21)/t10-,11-,12+,13+,16-,17-,18-,19-/m1/s1
|
|
| 化学名 |
4-oxo-4-(((3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-trimethyldecahydro-12H-3,12-epoxy[1,2]dioxepino[4,3-i]isochromen-10-yl)oxy)butanoic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (5.41 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.41 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (5.41 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6013 mL | 13.0066 mL | 26.0132 mL | |
| 5 mM | 0.5203 mL | 2.6013 mL | 5.2026 mL | |
| 10 mM | 0.2601 mL | 1.3007 mL | 2.6013 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。