| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Abl1 (IC50 = 2.8 nM); TrkA (IC50 = 6 nM); Abl1 (IC50 = 2.8 nM); TrkB (IC50 = 9 nM); Tie-2 (IC50 = 22 nM); Aurora B (IC50 = 98 nM)
|
|---|---|
| 体外研究 (In Vitro) |
盐酸阿西米尼 (ABL001) 将自身附着在 ABL1 的肉豆蔻酰口袋上,并导致激酶构象变得失活[1]。
盐酸阿西米尼选择性有效地与 ABL1 的肉豆蔻酰口袋结合,诱导失活的 C 末端螺旋构象(解离常数) :0.5-0.8 nM)。盐酸阿西米尼的效力大约高出 100 倍,与 BCR-ABL 抑制剂 GNF-2[1] 具有相同的非 ATP 竞争性生化动力学。 盐酸阿西米尼对 G 蛋白偶联受体、离子通道、核受体、转运蛋白以及超过 60 种激酶,包括 SRC[1]。 Asciminib 盐酸盐在不含 IL 的 BCR-ABL1 转化 Ba/F3 细胞中的抗增殖活性的 IC50 值为 0.25 nM -3。一小时后,盐酸阿西米尼的浓度与抑制细胞增殖所需的浓度相关,会磷酸化 CML 急变期细胞系 KCL-22 中的 STAT5 (Tyr694; pSTAT5) 和 BCR–ABL1 (Tyr245; pBCR–ABL1) [1]. 盐酸阿西米尼对每种 BCR-ABL1 细胞系均具有特异性活性(IC50 值为 1–20 nM),无论是否存在 p210 或 p190 BCR–ABL1 同工型[1]。 |
| 体内研究 (In Vivo) |
阿西米尼必须以至少 7.5 mg/kg 每天两次 (BID) 或 30 mg/kg 每天一次 (QD) 的剂量给予植入 KCL-22 肿瘤的小鼠才能完全消退。耐受剂量高达 250 mg/kg BID[1]。携带 KCL-22 异种移植物的小鼠接受单剂量的 Asciminib(7.5、15 和 30 mg/kg),其抑制 pSTAT5 (Tyr694)。剂量逆转发生在初次给药后 10、12 和 16-20 小时。
|
| 酶活实验 |
ABL1生化激酶测定[1]
在Sf21细胞中与YopH共表达产生ABL1 WT (64-515aa)蛋白。细胞离心后重悬于25mM Tris pH 7.0、500 mM NaCl、5%甘油、10 mM咪唑、1x完全蛋白酶抑制剂片、Benzonase (1:10 000 v:v)和1 mM TCEP中。用浆液均质法裂解细胞,离心清除细胞。ABL1 WT (64- 515aa)采用Ni-SepharoseFF柱亲和层析纯化,使用上述重悬浮缓冲液(分别含有10 mM和35mM咪唑)进行两次连续洗涤,并在含有250 mM咪唑的缓冲液中洗脱。将含有ABL1的馏分混合并上载到预平衡的SEC柱上,溶液为25 mM Tris pH 7.0、200 mM NaCl、5%甘油和1 mM TCEP。采用DELFIA®TRF法检测酶活性和化合物抑制作用。反应混合物含有500 nM Biotin-EAIYAAPFAKKK肽,10或2000µM ATP和25 pM ABL1 WT (64-515 aa)酶,反应缓冲液含有50 mM HEPES pH 7.2, 10 mM MgCl2, 2 mM DTT和0.01% Triton-X100。反应在60µL的体积中进行40 min,用20µL 500 mM EDTA(终浓度125 mM)淬火。将50µL的反应液转移到neutroavidin包被的384孔板上,室温下振荡孵育1小时。用100µL/孔TBST缓冲液洗涤后,加入50µL/孔Eu-anti-p-Tyr, 4℃摇瓶孵育过夜。加入50µL/孔DELFIA®增强液,在室温下孵育5分钟。在EnVision上使用时间分辨荧光Ex/Em: 340/615 nm读取板。对于抑制研究,化合物在DMSO中连续稀释,使用16点3倍格式,从5毫米的最高浓度。然后通过声传递系统将系列稀释化合物每孔100 nL转移到Grenier聚丙烯v底384孔分析板上。DMSO终浓度为0.16%,抑制剂终浓度为50µM ~ 3.48E-6µM。每个化合物重复测试,使用GraphPad Prism v6.02基于对照归一化的归一化IC50回归曲线拟合分析抑制剂的剂量响应曲线。
|
| 细胞实验 |
Ba/F3增殖试验[1]
对于每个细胞系,细胞密度调整为50 000个细胞/ml, 384孔检测板每孔加50ul(2500个细胞)。将测试化合物以10mM浓度的二甲基亚砜重悬。在384孔板中使用Janus液体分配器对每种化合物用DMSO进行连续三倍稀释。2nL化合物通过ATS-100 (EDC)的声传递以50µL的体积递送到含有2500个细胞的检测板中。细胞与化合物在37°C加5%二氧化碳的潮湿环境中孵育48小时。根据制造商的说明配制Britelite plus溶液,每孔加入25µl。培养皿孵育7分钟,在EnVision多模读板仪上检测发光。发光的程度与每孔中细胞的数量有关。因此,可以计算出每种抑制剂浓度的影响,并生成IC50。
|
| 动物实验 |
ABL001 (free base, solid dispersion form) was suspended in phosphate-buffered saline. Dosing solutions were prepared fresh every 3-4 days for dosing. ABL001 (free base, solution form) was formulated in 30% PEG 300, 6% Solutol HS15 in an acidic buffered solution. Dosing solutions were freshly prepared weekly for dosing.
Efficacy studies [3] For efficacy studies in subcutaneous KCL-22 xenograft model, mice bearing tumors of 100- 300mm3 were randomized into treatment groups (n=6 per group) for daily compound treatment. Body weight and tumor volume were recorded twice weekly for the duration of each study. In ABL001 dose-response studies, studies were terminated when vehicle-treated animals reached 1500mm3 mean tumor volume. In ABL001 and nilotinib combination efficacy study, select randomized groups animals were dosed daily with either ABL001 or nilotinib as single agents until tumor relapse (tumor volume >500mm3), then switched to the other agent continuously until second relapse. Animals are terminated as their final tumor volume reached >600mm3 . Another randomized group received combination of both ABL001 and nilotinib daily treatment then continued monitoring post-treatment cessation. For efficacy studies in systemic primary Ph+ ALL xenograft models, mice were injected intravenously with 5x106 ALL cells. Blood was sampled weekly from tail snip to monitor tumor burden, and engrafted mice with >10% human CD45+ cells were randomized into treatment groups for compound treatment (n=6 mice per group). Pharmacokinetics (PK) / Pharmacodynamics (PD) studies [3] Baseline tumor PD samples were collected from KCL-22 xenografts by fine needle biopsy before drug treatment. Animals received a single oral dose of ABL001 at 7.5 – 30 mg/kg. Blood was collected by serial tail bleed at designated time points (1-20h) for plasma PK analyses, and matching tumor PD samples were collected by fine needle biopsy at the same timepoints. |
| 药代性质 (ADME/PK) |
Absorption
The median Tmax of asciminib following oral administration is 2.5 hours. At a dose of 80mg once daily, the steady-state Cmax and AUCtau were 1781 ng/mL and 15112 ng.h/mL, respectively. At a dose of 40mg twice daily, the steady-state Cmax and AUCtau were 793 ng/mL and 5262 ng.h/mL, respectively. At a dose of 200mg twice daily (for treatment of T315I mutants), the steady-state Cmax and AUCtau were 5642 ng/mL and 37547 ng.h/mL, respectively. As compared to the fasted state, the co-administration of asciminib with a high-fat meal decreased the AUC and Cmax by 62% and 68%, respectively, and its co-administration with a low-fat meal decreased the AUC and Cmax by 30% and 35%, respectively. Route of Elimination Asciminib is eliminated via biliary secretion facilitated by breast cancer-resistant protein (BCRP) transporters. Following oral administration, approximately 80% and 11% of an asciminib dose was recovered in the feces and urine, respectively. Unchanged parent drug accounted for 57% of drug material recovered in the feces and 2.5% in the urine. Volume of Distribution At steady-state, the apparent volume of distribution of asciminib is 151 L. Clearance The total apparent clearance of asciminib is 6.7 L/h at a total daily dose of 80mg and 4.1 L/h at a dose of 200mg twice daily. Metabolism / Metabolites Asciminib is negligibly metabolized, with unchanged parent drug comprising the main drug component in plasma (~93%) and following excretion (~57% in feces). The main circulating metabolites are M30.5, M44, and M29.5, accounting for approximately 5%, 2%, and 0.4% of the total administered dose, respectively. The oxidative metabolism of asciminib is mediated by CYP3A4, and the glucuronidation of asciminib is mediated by UGT2B7 and UGT2B17. Biological Half-Life The terminal elimination half-life asciminib is 5.5 hours when administered at 40mg twice daily and 9.0 hours when administered at 200mg twice daily. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In the prelicensure clinical trials of asciminib in patients with refractory and extensively treated CML, ALT elevations arose in 13% of patients but were usually self-limited and mild. ALT elevations above 5 times the upper limit of normal (ULN) were uncommon, being found in 3% of treated patients. The ALT elevations were typically transient and rarely required dose interruption or modification. In the open label and controlled trials supporting the approval of asciminib, there were no instances of clinically apparent liver injury, hepatic failure or deaths from liver injury. Furthermore, patients with aminotransferase elevations during therapy with first and second line BCR-ABL1 inhibitors did not have an increased rate of such elevations during asciminib therapy. Since its approval in the United States and Europe, there have been no reported cases of clinically apparent liver injury associated with asciminib therapy. Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury). Protein Binding _In vitro_, asciminib is 97% bound to plasma proteins, although the specific protein(s) to which it binds are unclear. |
| 参考文献 | |
| 其他信息 |
Asciminib Hydrochloride is the hydrochloride salt form of asciminib, an orally bioavailable, allosteric Bcr-Abl1 tyrosine kinase inhibitor, with antineoplastic activity. Upon administration, asciminib targets and binds to the myristoyl pocket of the Bcr-Abl1 fusion protein at a location that is distinct from the ATP-binding domain, thereby inhibiting the activity of both wild-type Bcr-Abl and certain mutation forms, including the T315I mutation. This binding results in the inhibition of Bcr-Abl1-mediated proliferation and enhanced apoptosis of Philadelphia chromosome-positive (Ph+) hematological malignancies. The Bcr-Abl1 fusion protein tyrosine kinase is an abnormal enzyme produced by leukemia cells that contain the Philadelphia chromosome.
See also: Asciminib (has active moiety). Drug Indication Scemblix is indicated for the treatment of adult patients with Philadelphia chromosome positive chronic myeloid leukaemia in chronic phase (Ph+ CML CP) previously treated with two or more tyrosine kinase inhibitors (see section 5. 1). |
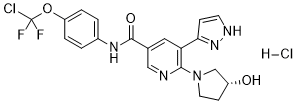
| 分子式 |
C20H19CL2F2N5O3
|
|---|---|
| 分子量 |
486.30
|
| 精确质量 |
485.083
|
| 元素分析 |
C, 49.40; H, 3.94; Cl, 14.58; F, 7.81; N, 14.40; O, 9.87
|
| CAS号 |
2119669-71-3
|
| 相关CAS号 |
Asciminib;1492952-76-7
|
| PubChem CID |
133082086
|
| 外观&性状 |
White to off-white solid powder
|
| tPSA |
103Ų
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
32
|
| 分子复杂度/Complexity |
626
|
| 定义原子立体中心数目 |
1
|
| SMILES |
C1CN(C[C@@H]1O)C2=C(C=C(C=N2)C(=O)NC3=CC=C(C=C3)OC(F)(F)Cl)C4=CC=NN4.Cl
|
| InChi Key |
HGCOOPLEWPBLOY-PFEQFJNWSA-N
|
| InChi Code |
InChI=1S/C20H18ClF2N5O3.ClH/c21-20(22,23)31-15-3-1-13(2-4-15)26-19(30)12-9-16(17-5-7-25-27-17)18(24-10-12)28-8-6-14(29)11-28;/h1-5,7,9-10,14,29H,6,8,11H2,(H,25,27)(H,26,30);1H/t14-;/m1./s1
|
| 化学名 |
N-[4-[chloro(difluoro)methoxy]phenyl]-6-[(3R)-3-hydroxypyrrolidin-1-yl]-5-(1H-pyrazol-5-yl)pyridine-3-carboxamide;hydrochloride
|
| 别名 |
asciminib; Asciminib hydrochloride; ABL-001; ABL001; Asciminib hydrochloride; Scemblix; ABL001-AAA; 2119669-71-3; Asciminib HCl; C5U34S9XFV; UNII-C5U34S9XFV; Asciminib hydrochloride [USAN]; AB -001; ABL001-AAA
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~100 mg/mL (~205.6 mM)
H2O: <0.1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.14 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.14 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.14 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0563 mL | 10.2817 mL | 20.5634 mL | |
| 5 mM | 0.4113 mL | 2.0563 mL | 4.1127 mL | |
| 10 mM | 0.2056 mL | 1.0282 mL | 2.0563 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04795427 | Active Recruiting |
Other: best available treatment Drug: asciminib |
Leukemia, Chronic Myelogenous | Novartis Pharmaceuticals | December 6, 2021 | Phase 2 |
| NCT04948333 | Active Recruiting |
Drug: ABL001 40mg BID Drug: ABL001 80mg QD |
Chronic Myelogenous Leukemia | Novartis Pharmaceuticals | October 13, 2021 | Phase 3 |
| NCT03906292 | Active Recruiting |
Drug: Imatinib Drug: Asciminib |
Chronic Myeloid Leukemia | University of Jena | August 19, 2019 | Phase 2 |
| NCT03106779 | Active Recruiting |
Drug: Bosutinib Drug: Asciminib |
Chronic Myelogenous Leukemia | Novartis Pharmaceuticals | October 26, 2017 | Phase 3 |
| NCT05943522 | Recruiting | Other: Asciminib | Chronic Myeloid Leukemia | Novartis Pharmaceuticals | July 19, 2023 |