| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
ETA ( IC50 = 0.055 nM )
Atrasentan (ABT-627, 0-50 μM) strongly inhibits the growth of LNCaP and C4-2b prostate cancer cells. When combined with Taxotere, ABT-627 causes a notably higher reduction in viable prostate cancer cells compared to when either drug is used alone. It also exhibits a higher degree of NF-κB DNA binding activity down-regulation[2]. Atrasentan significantly induces a number of CYPs and drug transporters (CYP3A4 is 12-fold induced at 50 μM, for example). It is a weak BCRP inhibitor (IC50 in MDCKII-BCRP cells = 59.8±11 μM) and a moderate P-gp inhibitor (IC50 in P388/dx cells = 15.1±1.6 μM)[3]. |
|---|---|
| 体外研究 (In Vitro) |
Atrasentan (ABT-627, 0-50 μM) 显着抑制 LNCaP 和 C4-2b 前列腺癌细胞生长。与单独使用任一药物相比,ABT-627 与泰索帝联合使用可显着增加活前列腺癌细胞的损失,并显示出更大程度的 NF-κB DNA 结合活性下调[2]。 Atrasentan 可显着诱导多种 CYP 和药物转运蛋白(例如 50 μM 时可诱导 12 倍 CYP3A4)。它是一种中度 P-gp 抑制剂(P388/dx 细胞中的 IC50=15.1±1.6 μM)和弱 BCRP 抑制剂(MDCKII-BCRP 细胞中的 IC50=59.8±11 μM)[3]。
Atrasentan HCl (ABT-627) 处理 (0-50 μM, 72小时) 以剂量依赖性方式降低雄激素受体 (AR) 阳性的LNCaP (10, 25, 50 μM下分别减少18%, 30%, 60%) 和C4-2b (10, 25, 50 μM下分别减少15%, 32%, 56%) 前列腺癌细胞的活力,但对AR阴性的PC-3细胞无效。 [2] Atrasentan HCl (25 μM) 与多西他赛 (Taxotere, 1 nM) 联用72小时,相比单药治疗 (~40% 抑制率),在LNCaP和C4-2b细胞中引起显著更强的生长抑制 (60-70% 抑制率) 和细胞凋亡诱导。该组合对PC-3细胞无效。 [2] 用Atrasentan HCl (5-25 μM) 处理C4-2b细胞,以剂量和时间依赖性方式下调了组成型核因子κB (NF-κB) 的DNA结合活性,与多西他赛联用时下调更显著。 [2] C4-2b细胞的Western印迹分析显示,Atrasentan HCl (25 μM) 和多西他赛 (1 nM) 联合处理72小时,相比单药治疗,导致更强的PARP剪切以及抗凋亡蛋白 (Bcl-2, Bcl-xL, survivin) 和磷酸化Akt的下调。 [2] 流式细胞术分析显示,C4-2b细胞经Atrasentan HCl (25 μM) 和多西他赛 (1 nM) 联合处理48小时后,亚G0-G1期凋亡细胞群比单用多西他赛增加了2.88倍。 [2] |
| 体内研究 (In Vivo) |
Atrasentan (3 mg/kg, po) 抑制大内皮素-1 (1 nmol/kg) 在髓大鼠中诱导的升压反应[1]。在 SCID-hu 模型中,Aatrasentan(ABT-627,10 mg/kg,腹腔注射)以及单独的泰索帝在一定程度上抑制了骨环境中 C4-2b 肿瘤的生长[2]。
在前列腺癌骨转移SCID-hu小鼠模型 (C4-2b细胞) 中,在检测到肿瘤后开始联合治疗:Atrasentan HCl (10 mg/kg,腹腔注射,每日一次,持续5周) 和多西他赛 (5 mg/kg,静脉注射,每3天一次,共4剂)。治疗5周后,与未治疗对照组相比,联合治疗抑制了90%的肿瘤生长。单药治疗也抑制生长,但程度较轻。 [2] 所有治疗组的小鼠血清前列腺特异性抗原 (PSA) 水平均显著降低,与肿瘤体积减小一致。 [2] 任何治疗组均未观察到显著的体重减轻,表明在该实验条件下无主要治疗相关毒性。 [2] 对收获的肿瘤组织分析显示,联合治疗组中NF-κB DNA结合活性及其下游靶点survivin和Bcl-2的表达显著下调,证实了体外结果。 [2] 联合治疗组肿瘤的组织病理学评估显示,部分样本中肿瘤细胞出现明显的胞浆空亮和空泡化,形成较小的肿瘤巢伴致密纤维化,骨碎片边缘成骨细胞反应不明显。 [2] |
| 细胞实验 |
所有三种前列腺癌细胞系(LNCaP、C4-2b 和 PC-3 细胞)均以每孔 3 × 103 个细胞的密度接种在 96 孔微量滴定培养板中。过夜孵育后,除去培养基并更换为含有从 10 mM 库存稀释的不同浓度 ABT-627 (0-50 μM) 的新鲜培养基。与药物孵育 72 小时后,向每孔中添加 20 μL MTT 溶液(PBS 中 5 mg/mL)并进一步孵育 2 小时。终止后,吸出上清液,并将代谢活细胞形成的 MTT 甲臜溶解在异丙醇 (100 μL) 中。将板在旋转摇床上混合 30 分钟,并在读板器上测量 595 nm 处的吸光度。
对于细胞活力 (MTT) 实验,将前列腺癌细胞系 (LNCaP, C4-2b, PC-3) 以每孔3x10³个细胞接种于96孔板。过夜孵育后,用递增浓度的Atrasentan HCl (0-50 μM) 或与多西他赛 (1 nM) 联合处理72小时。加入MTT溶液 (5 mg/mL,20 μL/孔),孵育2小时。形成的甲瓒晶体用异丙醇 (100 μL/孔) 溶解,在595 nm波长下测量吸光度。 [2] 对于ELISA法检测凋亡,用Atrasentan HCl (25 μM) 和/或多西他赛 (1 nM) 处理LNCaP和C4-2b细胞72小时。按照试剂盒说明书提取胞浆组蛋白/DNA片段并进行检测,在405 nm波长下测量吸光度。 [2] 对于流式细胞术分析凋亡,用Atrasentan HCl (25 μM)、多西他赛 (1 nM) 或其组合处理C4-2b细胞48小时。收集细胞 (包括悬浮和贴壁细胞),用75%乙醇固定,碘化丙啶和RNase A染色,进行流式细胞术分析。计算亚G0-G1期的凋亡细胞百分比。 [2] 对于Western印迹分析,用Atrasentan HCl (25 μM) 和/或多西他赛 (1 nM) 处理C4-2b细胞72小时。裂解细胞,蛋白质 (50 μg) 经SDS-PAGE分离,转膜,并用特异性抗体 (如PARP, Bcl-2, Bcl-xL, survivin, Akt, p-Akt) 进行检测。使用增强化学发光试剂盒进行显色。 [2] 对于Akt激酶活性实验,处理C4-2b细胞并裂解。使用固定的Akt抗体从裂解液 (150 μg蛋白) 中免疫沉淀Akt。将免疫沉淀物与ATP和GSK-3α/β融合蛋白底物在30°C下孵育30分钟。通过Western印迹检测GSK-3α/β的磷酸化。 [2] 对于电泳迁移率变动分析 (EMSA),从处理过的C4-2b细胞中制备核蛋白提取物。提取物 (8 μg) 与IRDye-700标记的NF-κB寡核苷酸探针孵育。DNA-蛋白复合物在非变性聚丙烯酰胺凝胶上分离,并使用红外成像系统显像。 [2] |
| 动物实验 |
Rats are orally given YM598 (0.3, 1, and 3 mg/kg), atrasentan (0.3, 1, and 3 mg/kg), or 0.5% methyl cellulose as a vehicle using a dosing cannula. 5 mL/kg is the dosage volume for both the test material and the vehicle. The rats are anesthetized with NSC 10816 about 20 minutes after the compounds are administered, and 30 minutes after dosing, they are pithed and ventilated. Big endothelin-1 (1 nmol/kg) is injected intravenously and blood pressure is recorded about an hour after the compounds are taken orally. In these two experiments, linear regression analysis is used to determine the dose of test compound that causes 50% inhibition (ID50) of the big endothelin-1-induced increase in diastolic blood pressure.
Male homozygous CB-17 SCID/SCID mice (4 weeks old) were implanted with a single human fetal bone fragment. C4-2b prostate cancer cells (1x10⁶ in 20 μL serum-free medium) were injected intraosseously into the implanted bone. [2] Treatment began when bone implants showed signs of enlargement (~30 days post-injection). Mice were randomized into groups (n=7): untreated control; Atrasentan HCl alone (10 mg/kg, i.p., daily for 5 weeks); Taxotere alone (5 mg/kg, i.v., every 3rd day for 4 doses); combination (Atrasentan HCl + Taxotere, same schedules). Tumor volume was measured twice weekly. [2] Mice were euthanized one day after the last Atrasentan HCl dose (5 weeks). Tumors were excised, measured, and processed for histology (H&E staining) and molecular analysis (nuclear protein extraction for EMSA, Western blot). Serum was collected for PSA measurement by ELISA. [2] For tumor tissue nuclear protein extraction and EMSA, harvested tumors were minced and homogenized in ice-cold buffer. Nuclear pellets were suspended in high-salt extraction buffer, incubated on ice, and centrifuged. Supernatants containing nuclear proteins were collected for EMSA analysis as described in the Cell Assay section. [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
No significant body weight loss or obvious treatment-related toxicity was observed in SCID-hu mice treated with Atrasentan HCl alone (10 mg/kg/day, i.p., 5 weeks), Taxotere alone (5 mg/kg, i.v., q3d, 4 doses), or their combination. [2]
|
| 参考文献 |
|
| 其他信息 |
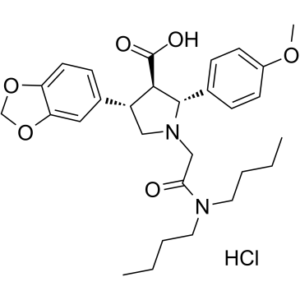
Atrasentan Hydrochloride is the orally available hydrochloride salt of pyrrolidine-3-carboxylic acid with potential antineoplastic activity. As a selective antagonist of the endothelin-A (ETA) receptor, atrasentan binds selectively to the ETA receptor, which may result in inhibition of endothelin-induced angiogenesis and tumor cell proliferation.
A pyrrolidine and benzodioxole derivative that acts a RECEPTOR, ENDOTHELIN A antagonist. It has therapeutic potential as an antineoplastic agent and for the treatment of DIABETIC NEPHROPATHIES. Drug Indication Treatment of nephropathy Atrasentan HCl (ABT-627) is a potent, orally bioavailable, and selective ETA receptor antagonist. Endothelin-1 (ET-1), produced by prostate cancer cells, binds to ETA receptors on bone marrow stromal cells, promoting osteoblastic response during bone metastasis. Blocking this interaction may inhibit bone metastasis and enhance the efficacy of docetaxel (Taxotere). [2] The proposed mechanism involves ET-1 activating the PI3K-Akt pathway, leading to NF-κB activation and upregulation of anti-apoptotic genes (e.g., Bcl-2, survivin). Atrasentan HCl inhibits this pathway, downregulating NF-κB and sensitizing cancer cells to Taxotere-induced apoptosis. [2] The SCID-hu model used mimics the human bone metastatic microenvironment, showing both osteoblastic and osteolytic lesions similar to clinical disease. [2] Phase III clinical trials indicated that Atrasentan significantly delayed disease progression in men with prostate cancer bone metastases compared to placebo. [2] |
| 分子式 |
C29H39CLN2O6
|
|---|---|
| 分子量 |
547.08276
|
| 精确质量 |
546.25
|
| 元素分析 |
C, 63.67; H, 7.19; Cl, 6.48; N, 5.12; O, 17.55
|
| CAS号 |
195733-43-8
|
| 相关CAS号 |
Atrasentan; 173937-91-2
|
| PubChem CID |
159595
|
| 外观&性状 |
Off-white to gray solid powder
|
| 密度 |
1.238g/cm3
|
| 沸点 |
659.4ºC at 760mmHg
|
| 闪点 |
352.6ºC
|
| LogP |
5.433
|
| tPSA |
88.54
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
12
|
| 重原子数目 |
38
|
| 分子复杂度/Complexity |
734
|
| 定义原子立体中心数目 |
3
|
| SMILES |
O=C([C@H]1[C@H](C2=CC=C(OC)C=C2)N(CC(N(CCCC)CCCC)=O)C[C@@H]1C3=CC=C(OCO4)C4=C3)O.Cl
|
| InChi Key |
IJFUJIFSUKPWCZ-SQMFDTLJSA-N
|
| InChi Code |
InChI=1S/C29H38N2O6.ClH/c1-4-6-14-30(15-7-5-2)26(32)18-31-17-23(21-10-13-24-25(16-21)37-19-36-24)27(29(33)34)28(31)20-8-11-22(35-3)12-9-20;/h8-13,16,23,27-28H,4-7,14-15,17-19H2,1-3H3,(H,33,34);1H/t23-,27-,28+;/m1./s1
|
| 化学名 |
(2R,3R,4S)-4-(1,3-benzodioxol-5-yl)-1-[2-(dibutylamino)-2-oxoethyl]-2-(4-methoxyphenyl)pyrrolidine-3-carboxylic acid;hydrochloride
|
| 别名 |
ABT 627; Abbott 147627; (+)A 127722; A147627; A 127722; ABT627; ABT-627; NSC720763; US trade name: Xinlay
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~28.57 mg/mL (~52.2 mM)
H2O: ~0.5 mg/mL (~0.9 mM) 0.1 M HCL: < 1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.57 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.57 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.57 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 0.75 mg/mL (1.37 mM) in 0.5% CMC-Na/saline water (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 通过加热和超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8279 mL | 9.1394 mL | 18.2789 mL | |
| 5 mM | 0.3656 mL | 1.8279 mL | 3.6558 mL | |
| 10 mM | 0.1828 mL | 0.9139 mL | 1.8279 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00046943 | Completed | Drug: atrasentan hydrochloride | Prostate Cancer | Abbott | September 2002 | Phase 3 |
| NCT00039429 | Completed | Drug: atrasentan hydrochloride | Kidney Cancer | Eastern Cooperative Oncology Group | July 14, 2003 | Phase 2 |
| NCT00134056 | Completed | Drug: atrasentan hydrochloride Drug: docetaxel |
Metastatic Cancer Prostate Cancer |
SWOG Cancer Research Network | August 2006 | Phase 3 |
| NCT02118714 | Completed | Drug: Atrasentan | Nephropathy Diabetes |
AbbVie | April 6, 2015 | Phase 2 |
| NCT00181558 | Completed | Drug: Atrasentan Drug: Zoledronic Acid (Zometa) |
Adenocarcinoma of the Prostate Prostate Cancer |
Massachusetts General Hospital | December 2001 | Phase 2 |