| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Growth hormone; Microbial Metabolite; Endogenous Metabolite
|
|---|---|
| 体外研究 (In Vitro) |
二次发酵阶段对于稳定堆肥产品和产生各种次生代谢物至关重要。然而,嗜中温菌的低代谢率被认为是堆肥过程中的限速阶段。在本研究中,接种了两种产吲哚乙酸(IAA)的细菌(安全芽孢杆菌33C和安全棒杆菌29B),以加强二次发酵阶段,提高堆肥产品的植物生长促进潜力。结果表明,添加IAA产生菌促进了可溶性盐的同化、腐殖质的缩合和芳构化,以及溶解有机氮(DON)和溶解有机碳(DOC)的积累。生物强化策略还使二次发酵中后期的微生物群落演替更快。然而,芽孢杆菌和棒杆菌的定殖并不能解释IAA产量的不成比例的增加,与对照组相比,IAA产量高达5.6倍。结合理化性质和微生物群落结构的深入分析表明,产生IAA的细菌可能会导致盐度的增加,从而丰富了能够产生IAA的耐盐细菌,如嗜盐单胞菌、短杆菌和黄杆菌。此外,研究结果还证明,有必要缩短二次发酵时间,以避免IAA降解,同时不影响堆肥成熟度。综上所述,通过添加适当的IAA产生菌来增强堆肥的二次发酵是提高有机肥料质量的有效策略。[1]
细胞质雄性不育(CMS)为杂种优势的商业开发和高产杂交水稻的生产提供了不可替代的策略。外源施用植物生长调节剂可以通过影响花性状来提高CMS系的异交率,从而增加杂交水稻种子产量。本研究旨在探讨赤霉素(GA3)、3-吲哚乙酸(IAA)和萘乙酸(NAA)等生长调节剂对促进不同水稻CMS系的花性状和异交率以及提高杂交水稻种子产量的影响。研究了叶面施用包含300 g/ha GA3或150 g/ha GA3+50 g/ha+200 g/ha NAA的生长调节剂与未处理对照对五种不同CMS品系的花、生长和产量性状的影响。外源喷洒的生长调节剂,特别是GA3、IAA和NAA(T3)的组合,促进了所有测试的CMS系中所有研究的花、生长和产量性状。此外,所评估的CMS品系在所有测量的花性状上都表现出显著差异。L2、L3和L1显示了最上部小穗开放角度、小穗开放持续时间、柱头总长度、花柱长度、柱头刷和柱头宽度。此外,这些CMS品系表现出最高的植物生长和产量性状,特别是在T3下。因此,外源施用GA3、IAA和NAA可以改善L2、L3和L1等有前景的CMS系的花、生长和产量性状,从而提高异交率和杂交水稻种子产量。[2] 3-吲哚乙酸(IAA)是一种植物生长调节剂,在植物生长发育中起着重要作用,参与非生物胁迫的调节。为了探讨IAA对樟树镉毒性的影响,对一年生樟树幼苗进行了室内盆栽试验。分析了在添加或不添加10mg kg-1 IAA的情况下,IAA对用30mg kg-1镉处理的樟树叶片中镉积累、净光合速率、呼吸、光合色素(叶绿素a、叶绿素b、总叶绿素和类胡萝卜素)、渗透调节物质(脯氨酸、可溶性糖和可溶性蛋白)和丙二醛含量的影响。添加外源IAA的樟树叶片中镉的积累显著高于不添加IAA的镉胁迫下的积累(60天后约为69.10%)。在培养期间,不添加IAA的镉胁迫下,樟树叶片的净光合速率比对照植物低24.31%。镉胁迫和添加IAA的樟树叶片的净光合速率比未添加IAA的镉胁迫叶片高出30.31%。未添加IAA的镉胁迫叶片中叶绿素a、总叶绿素和类胡萝卜素含量低于对照处理。与不添加IAA的镉胁迫相比,IAA的存在增加了叶绿素a、总叶绿素和类胡萝卜素含量。在不添加IAA的情况下,镉胁迫下樟树叶片的呼吸速率和脯氨酸、可溶性糖、可溶性蛋白和丙二醛的浓度均高于对照组。与未添加IAA的镉胁迫相比,添加IAA降低了樟树叶片的呼吸速率以及脯氨酸、可溶性糖、可溶性蛋白和丙二醛的浓度。这些结果表明,外源IAA通过提高净光合速率、增加渗透调节物质浓度、去除活性氧自由基和消除潜在损伤来改善樟树的光合性能和生长环境,从而减少镉对樟树的毒性作用[3]。 |
| 体内研究 (In Vivo) |
本研究旨在调查3-Indoleacetic acid/3-吲哚乙酸(IAA)对大鼠血液学参数、肝肾功能、心肌和骨骼肌以及睾丸的可能不利影响,以及各器官的组织病理学改变,并确定IAA停药后动物发生的任何不利影响的逆转程度。大鼠通过胃插管每天一次口服500mg/kg BW,持续14天,之后处死一半,另一半再放置14天,不接触IAA。大鼠接触IAA会导致贫血、白细胞减少、中性粒细胞减少、淋巴细胞减少,血清转氨酶、γ-谷氨酰转移酶、肌酸激酶心肌带、肌酸激酶肌型和血清肌酐、钠、氯和钾水平显著升高。此外,血清睾酮、促性腺激素和瘦素水平显著下降。IAA停药后,大多数测量参数的变化仍在继续。不同组织的组织病理学改变支持这些变化。总之,高浓度IAA的亚急性暴露会对许多软器官产生血液毒性和毒性作用,停药会导致动物不完全恢复。因此,应谨慎使用IAA,因为高浓度的广泛使用会对环境、动物和人类造成有害影响[4]。
|
| 细胞实验 |
本研究进行了三种处理,包括未受污染的土壤+樟脑(对照)、镉污染的土壤(30 mg kg−1)+樟脑(T1)和镉污染土壤(30 mgkg−l)+樟脑+IAA(10 mgkg−1)(T2)。每种处理都使用了五个重复。土壤中镉的浓度是根据生态环境部和国家市场监督管理总局联合发布的《土壤环境质量——开发用地土壤污染风险控制标准(GB3660-2018)》设定的。
制备了2 g L−1 CdCl2·2.5H2O(根据Cd2+浓度计算)和0.5 g L−13-吲哚乙酸/IAA溶液。土壤中镉和IAA的最终浓度分别为30 mg kg-1和10 mg kg-1,当镉和IAA浓度分别达到30 mg kg−1和10 mg公斤−1时种植幼苗。氮(300 kg ha−1)和磷(150 kg ha−1)通常作为水溶性肥料施用,这些肥料被稀释并作为基肥施用。盆栽植物被放置在光照测试台上;每盆直径15cm×高度24cm,含土2.5kg。锅的位置每周都会改变。称量花盆的重量,每天加入去离子水,使土壤湿度保持在饱和含水量的50%。在培养期间,光照(光强=8000-10000勒克斯)和黑暗期分别设置为12小时。在第0、15、30和60天对植物进行取样,并测量净光合速率、光合色素、渗透调节因子和MDA含量等生理生化指标[3]。
|
| 动物实验 |
Experimental procedure [4]
Thirty-six rats were divided randomly into three groups, with 12 rats in each group as follows: Group I (control): rats were given only standard feed and water. Group II (vehicle): rats orally received 0.5 ml olive oil orally by gastric intubation once daily for 14 days. Group III (IAA): rats orally received IAA/3-Indoleacetic acid powder suspended in olive oil at concentration of 500 mg/kg BW by gastric intubation once daily for 14 days. The dose of IAA was selected based on the previously published studies (Furukawa et al. 2004). Bearing that in mind, the median lethal dose (LD50) of IAA in rats (oral treatment) is more than 500 mg/kg BW (Paley 2021). After 14 days of IAA exposure, the rats were maintained for another 14 consecutive days without any treatment. Sampling [4] Sample collection during the experiment course occurred twice, after 14 days from exposure to 3-Indoleacetic acid/IAA and after 14 days of stopping exposure to IAA from the different experimental groups. Blood samples were collected from overnight fasted rats after being anesthetized by sodium pentobarbital by puncturing the retro-orbital venous sinus. The first part of blood specimens (1 ml) was collected in clean Wasserman tubes containing dipotassium salt of ethylenediamine tetraacetic acid (EDTA) for performing various hematological tests. The second part of blood specimens (1.5 ml) was collected in ordinary tubes and left to coagulate for centrifugation and separation of serum for performing the different biochemistry and hormonal assays. Rats were euthanized by decapitation after being anesthetized and the liver, kidneys, heart, skeletal muscle (extensor digitorum longus), and testes were excised quickly for histopathological studies. |
| 药代性质 (ADME/PK) |
Metabolism / Metabolites
Indole-3-acetic-acid has known human metabolites that include Indole-3-acetic-acid-O-glucuronide. Indoleacetic acid (IAA) is a breakdown product of tryptophan metabolism and is often produced by the action of bacteria in the mammalian gut. Some endogenous production of IAA in mammalian tissues also occurs. It may be produced by the decarboxylation of tryptamine or the oxidative deamination of tryptophan. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Uremic toxins such as indole-3-acetic acid are actively transported into the kidneys via organic ion transporters (especially OAT3). Increased levels of uremic toxins can stimulate the production of reactive oxygen species. This seems to be mediated by the direct binding or inhibition by uremic toxins of the enzyme NADPH oxidase (especially NOX4 which is abundant in the kidneys and heart) (A7868). Reactive oxygen species can induce several different DNA methyltransferases (DNMTs) which are involved in the silencing of a protein known as KLOTHO. KLOTHO has been identified as having important roles in anti-aging, mineral metabolism, and vitamin D metabolism. A number of studies have indicated that KLOTHO mRNA and protein levels are reduced during acute or chronic kidney diseases in response to high local levels of reactive oxygen species (A7869) |
| 参考文献 |
[1]. Inoculating indoleacetic acid bacteria promotes the enrichment of halotolerant bacteria during secondary fermentation of composting. J Environ Manage. 2022 Nov 15;322:116021.
[2]. Growth Regulators Improve Outcrossing Rate of Diverse Rice Cytoplasmic Male Sterile Lines through Affecting Floral Traits. Plants (Basel). 2022 May 12;11(10):1291. [3]. Effects of exogenous 3-indoleacetic acid and cadmium stress on the physiological and biochemical characteristics of Cinnamomum camphora. Ecotoxicol Environ Saf. 2020 Mar 15;191:109998. [4]. Assessment toxic effects of exposure to 3-indoleacetic acid via hemato-biochemical, hormonal, and histopathological screening in rats. Environ Sci Pollut Res Int. 2022 Dec;29(60):90703-90718. |
| 其他信息 |
Indole-3-acetic acid is a monocarboxylic acid that is acetic acid in which one of the methyl hydrogens has been replaced by a 1H-indol-3-yl group. It has a role as a plant hormone, a human metabolite, a plant metabolite, a mouse metabolite and an auxin. It is a monocarboxylic acid and a member of indole-3-acetic acids. It is a conjugate acid of an indole-3-acetate.
Indoleacetic acid is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). Indole-3-acetic acid has been reported in Humulus lupulus, Balansia epichloe, and other organisms with data available. Indoleacetic acid is a uremic toxin. Uremic toxins can be subdivided into three major groups based upon their chemical and physical characteristics: 1) small, water-soluble, non-protein-bound compounds, such as urea; 2) small, lipid-soluble and/or protein-bound compounds, such as the phenols and 3) larger so-called middle-molecules, such as beta2-microglobulin. Chronic exposure of uremic toxins can lead to a number of conditions including renal damage, chronic kidney disease and cardiovascular disease. Indoleacetic acid (IAA) is a breakdown product of tryptophan metabolism and is often produced by the action of bacteria in the mammalian gut. Some endogenous production of IAA in mammalian tissues also occurs. It may be produced by the decarboxylation of tryptamine or the oxidative deamination of tryptophan. IAA frequently occurs at low levels in urine and has been found in elevated levels in the urine of patients with phenylketonuria ( Using material extracted from human urine, it was discovered by Kogl in 1933 that Indoleacetic acid is also an important plant hormone Specifically IAA is a member of the group of phytohormones called auxins. IAA is generally considered to be the most important native auxin. Plant cells synthesize IAA from tryptophan. IAA and some derivatives can be oxidised by horseradish peroxidase (HRP) to cytotoxic species. IAA is only toxic after oxidative decarboxylation; the effect of IAA/HRP is thought to be due in part to the formation of methylene-oxindole, which may conjugate with DNA bases and protein thiols. IAA/HRP could be used as the basis for targeted cancer therapy involving antibody-, polymer-, or gene-directed approaches, a potential new role for plant auxins in cancer therapy. (A3268, A3269). indole-3-acetate is a metabolite found in or produced by Saccharomyces cerevisiae. Inoculating the secondary fermentation of composting with exogenous IAA-producing bacteria promoted the assimilation of soluble salt, the condensation and aromatization of humus, and the accumulation of IAA, DON and DOC. Microbial composition was mainly determined by the physicochemical properties and fermentation times, while the inoculation of IAA-producing bacteria accelerated the succession at the end. The massive accumulation of IAA in the early-medium phase of secondary fermentation could be highly associated with the enrichment of halotolerant bacteria, which might be induced by the increasing salinity caused by exogenous IAA-producing bacteria. Moreover, the proliferation of several IAA-degrading bacteria at the end of composting suggested the necessary to shorten secondary fermentation time in order to preserve more IAA without affecting the composting maturity. These results provide new ideas for optimizing aerobic composting technology.[1] Foliar application of gibberellic acid, indole-3-acetic acid, and naphthalene acetic acid in combination remarkably ameliorated all evaluated floral, growth, and yield characteristics of CMS lines compared to untreated plants. In addition, the evaluated CMS lines exhibited different genetic behavior for floral traits, plant growth, and plant productivity. L2 and L1 displayed the uppermost evaluated floral traits, plant growth, and yield traits, particularly under T3. Accordingly, it seems interesting to exploit foliar application of GA3, IAA, and NAA in combination as a useful tool in enhancing the outcrossing ability of promising CMS lines L2 and L1 to ameliorate outcrossing rates and hybrid seed production.[2] Cadmium stress reduced the net photosynthetic rate and photosynthetic pigment content in C. camphora leaves (chlorophyll a, total chlorophyll and carotenoids), enhanced the respiration rate, and increased quantities of proline, soluble sugars, soluble proteins and MDA. The addition of exogenous IAA improved the net photosynthetic rate in C. camphora leaves under cadmium stress, slowed respiration, reduced the proline and soluble sugar contents, alleviated cadmium-stress effects and promoted the accumulation of cadmium in C. camphora. In conclusion, the application of IAA can help C. camphora adapt to cadmium stress and increase cadmium accumulation. Therefore, C. camphora has potential for use in remediation of soils contaminated by heavy metals.[3] Considering the outcome of current study, I concluded that the subacute exposure to IAA at a high concentration could exert hematotoxicity appeared in form of a decrease in erythrogram parameters and leukopenia as well as hepatorenal dysfunction and various toxic effects on heart and testis in addition to skeletal muscles. The alterations in the hemato-biochemical and hormonal tests as well as the histological structure of different organs revealed these toxic effects. Also, the withdrawal of IAA resulted in incomplete recovery of animals from adverse impacts within the time course of the experimental investigation, so the recovery from such impacts could need more time. Thus, IAA should be used cautionary as extensive use of it in high concentrations can cause harmful effects on the environment, animals and human beings.[4] |
| 分子式 |
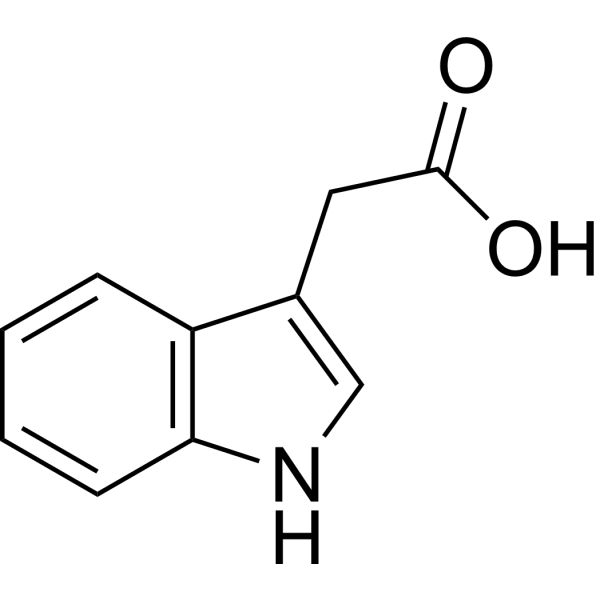
C10H9NO2
|
|---|---|
| 分子量 |
175.1840
|
| 精确质量 |
175.063
|
| 元素分析 |
C, 68.56; H, 5.18; N, 8.00; O, 18.27
|
| CAS号 |
87-51-4
|
| 相关CAS号 |
2338-19-4 (mono-potassium salt); 6305-45-9 (mono-hydrochloride salt); 6505-45-9
|
| PubChem CID |
802
|
| 外观&性状 |
Off-white to light brown solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
415.0±20.0 °C at 760 mmHg
|
| 熔点 |
165-169 °C(lit.)
|
| 闪点 |
204.8±21.8 °C
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
| 折射率 |
1.694
|
| LogP |
1.43
|
| tPSA |
53.09
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
13
|
| 分子复杂度/Complexity |
205
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C(CC1C2C(=CC=CC=2)NC=1)O
|
| InChi Key |
SEOVTRFCIGRIMH-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C10H9NO2/c12-10(13)5-7-6-11-9-4-2-1-3-8(7)9/h1-4,6,11H,5H2,(H,12,13)
|
| 化学名 |
2-(1H-indol-3-yl)acetic acid
|
| 别名 |
indole-3-acetic acid; 87-51-4; 3-Indoleacetic acid; indoleacetic acid; Heteroauxin; 1H-Indole-3-acetic acid; 1H-indol-3-ylacetic acid; 2-(1H-Indol-3-yl)acetic acid;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 30 mg/mL (~171.25 mM)
H2O : ~1 mg/mL (~5.71 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (11.87 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (11.87 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (11.87 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.7084 mL | 28.5421 mL | 57.0841 mL | |
| 5 mM | 1.1417 mL | 5.7084 mL | 11.4168 mL | |
| 10 mM | 0.5708 mL | 2.8542 mL | 5.7084 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。