| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
| 靶点 |
VEGFR1/FLT1 (IC50 = 0.1 nM); VEGFR2/Flk1 (IC50 = 0.18 nM); VEGFR2/KDR (IC50 = 0.2 nM); VEGFR3 (IC50 = 0.1 nM-0.3 nM nM); PDGFRβ (IC50 = 1.6 nM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:阿西替尼可以阻断 VEGFR 的细胞自磷酸化和 VEGF 介导的内皮细胞活力、管形成和下游信号传导。 Axitinib 抑制可变细胞系的增殖,IC50 >10,000 nM (IGR-N91)、849 nM (IGR-NB8)、274 nM (SH-SY5Y) 和 573 nM(非 VEGF 刺激的 HUVEC)。激酶测定:生成过表达全长 VEGFR2、PDGFRβ、Kit 的猪主动脉内皮 (PAE) 细胞和过表达鼠 VEGFR2 (Flk-1) 或 PDGFRα 的 NIH-3T3。 96孔板包被有100μL/孔的2.5μg/mL抗VEGFR2抗体、0.75μg/mL抗PDGFRβ抗体、0.25μg/mL抗PDGFRα抗体、0.5μg/mL抗KIT抗体,或1.20 μg/mL 抗 Flk-1 抗体用于制备 ELISA 捕获板。然后通过ELISA测量RTK的磷酸化。细胞测定:将细胞(HUVEC、SH-SY5Y、IGR-N91 和 IGR-NB8 细胞)以 5 × 104 的密度接种到 96 孔板中并培养 24 小时。阿西替尼以 1 nM 至 10 μM 的浓度添加到细胞中。 72小时后通过MTS四唑底物测量细胞活力并计算IC50值。
Axitinib有效抑制细胞VEGF RTK活性。[2] 在转染或内源性表达rtk的细胞中,阿西替尼能有效阻断生长因子刺激的VEGFR-2和VEGFR-3磷酸化,平均IC50值分别为0.2和0.1 ~ 0.3 nmol/L(图2A;表1)。针对VEGFR-1的细胞活性为1.2 nmol/L(在存在2.3%牛血清白蛋白的情况下测量),基于阿西替尼的蛋白质结合,相当于绝对IC50为~ 0.1 nmol/L。在Flk-1转染的NIH-3T3细胞中,对小鼠VEGFR-2 (Flk-1)的效价为0.18 nmol/L,与人同源物相似。阿西替尼对密切相关的III型和V型家族rtk,包括PDGFR-β (1.6 nmol/L), KIT (1.7 nmol/L)和PDGFR-α (5 nmol/L;表1);纳摩尔浓度的阿西替尼阻断PDGF bb介导的人胶质瘤U87MG细胞(PDGFR-β阳性)的迁移,但不增殖(数据未显示)。相比之下,阿西替尼对FGFR-1的靶标和功能活性要弱得多(图1A和B;表1)。当浓度高达1 μmol/L时,axitinib对RS;411细胞中的Flt-3和TT细胞中的RET的活性最小(数据未显示)。对上述受体的重组酪氨酸激酶的酶抑制活性(Ki)也观察到类似的趋势(数据未显示)。重要的是,阿西替尼对“脱靶”蛋白激酶几乎没有抑制作用;浓度为1 μmol/L (VEGFR-2的Ki的约1000倍),跨越约100个蛋白激酶的三个激酶组(辉瑞公司内部;Upstate和Dundee面板),阿西替尼仅抑制5种蛋白激酶:Abl、Aurora-2、Arg、AMPK、Axl和MST2(抑制率≥60%)。最后,阿西替尼在广泛的蛋白激酶筛选中没有表现出显著的活性(Cerep;数据未显示)。 Axitinib抑制vegf介导的内皮细胞存活、迁移和小管形成。[2] 阿西替尼对vegf刺激的HUVEC存活表现出强有力的抑制作用,但对基本的fgf刺激的HUVEC存活没有抑制作用,对VEGFR-2和FGFR-1受体的选择性约为1000倍(图2B)。功能实验得出的VEGFR-2的平均IC50值(0.24±0.09 nmol/L)与细胞受体磷酸化实验得出的IC50值相似(表1),证实受体拮抗导致该化合物的功能抑制。此外,阿西替尼剂量依赖性地抑制三维纤维蛋白基质系统中球体内皮管的形成(图2C;补充图S1)。由于系统中存在较高的血清水平(4-8% FBS),因此需要比其他类型的测定法更高的化合物浓度来抑制管的形成。 Axitinib抑制内皮细胞内信号转导。[2] Axitinib快速且剂量依赖性地降低了VEGF下游关键信号分子Akt、内皮一氧化氮合酶(eNOS)和细胞外信号调节激酶1/2 (ERK1/2)的磷酸化水平(图2D), IC50值与抑制VEGFR-2的IC50值相似。这表明Akt、eNOS和ERK1/2磷酸化的降低可能是由于阿西替尼对上游vegfr的拮抗作用。 |
| 体内研究 (In Vivo) |
阿西替尼对原位移植模型如 M24met(黑色素瘤)、HCT-116(结直肠癌)和 SN12C(肾细胞癌)表现出主要抑制作用。在 IGR-N91 侧腹异种移植物中,与对照组(口服 30 mg/kg)相比,阿西替尼延迟了肿瘤生长 11.4 天,并将平均血管密度 (MVD) 降低至 21,而对照组为 49。 10-100 mg/kg 的阿西替尼可显着抑制 BT474 乳腺癌模型的生长并破坏肿瘤微血管。阿西替尼已在多种肿瘤中表现出单药活性,包括肾细胞癌、甲状腺癌、非小细胞肺癌和黑色素瘤。
目前的研究旨在确定单独和联合治疗的病理生理后果,分别使用分割放疗加Axitinib/阿西替尼/AG-013736(一种优先抑制血管内皮生长因子受体的受体酪氨酸激酶抑制剂)。DU145人前列腺异种移植肿瘤分别用(a)载体单独治疗,(b)阿西替尼/Axitinib/AG-013736, (c) 5x2 Gy/wk放疗分数,或(d)联合治疗。使用免疫组织化学图像的自动图像处理来确定总血管和灌注血管间距,整体缺氧,周细胞/胶原覆盖,增殖和凋亡。与单药治疗相比,联合治疗产生了更高的肿瘤反应。血管密度逐渐下降,α -平滑肌肌动蛋白阳性周细胞覆盖率略有增加,整体肿瘤缺氧增加(与对照组相比)。尽管功能性血管内皮细胞凋亡选择性地增加,但总的血管和灌注血管的减少通常是成比例的,这表明联合治疗并不是专门针对功能性血管。这些结果反对AG-013736或联合治疗诱导的肿瘤血管功能正常化。血管消融表现为游离周细胞增多和IV型胶原空洞。尽管在3周的治疗中肿瘤氧合逐渐降低,但联合治疗仍然有效,肿瘤进展最小。[1] <人力资源> 阿西替尼/Axitinib在体内的靶调制。[2] 急性阿西替尼治疗迅速显著降低视网膜血管VEGFR-2磷酸化。第二次给药1小时后,与对照组织相比,视网膜VEGFR-2磷酸化降低了80%至90%(图3A,左)。给药后6和24 - 32 h, phospho-VEGFR-2水平分别恢复到对照组的50%和100%。在整个研究过程中,phospho-VEGFR-2水平与阿西替尼血浆浓度呈负相关。抑制VEGFR-2磷酸化的EC50值为0.49 nmol/L(或0.19 ng/mL,为血浆蛋白结合校正的未结合值);图3A,右)。[2] 阿西替尼/Axitinib还能抑制小鼠M24met异种移植肿瘤血管生成血管中VEGFR-2的磷酸化;M24met肿瘤分泌高VEGF-A,高度血管化,不表达功能性人VEGFR-2。与对照肿瘤相比,单次口服剂量的阿西替尼(100mg /kg)可显著抑制小鼠VEGFR-2磷酸化长达7小时(图3B)。下游ERK1/2的磷酸化也从相同的样品中测量。与对照组相比,早在给药后30分钟,在处理组织中就观察到ERK1/2信号的部分抑制,并且抑制至少持续7小时(图3C)。 [2] 阿西替尼/Axitinib快速抑制vegf诱导的小鼠皮肤血管通透性;小鼠的抑制作用呈剂量依赖性,与药物浓度直接相关(图3D)。药代动力学/药效学分析显示,未结合EC50为0.46 nmol/L(补充图S2)。在没有外源性VEGF-A刺激的MV522荷瘤小鼠皮肤中也显示出类似的抑制作用(数据未显示)。 [2] 综上所述,基于抑制血管VEGFR-2磷酸化和vegf介导的通透性所需的体内药理学浓度(Ctarget)为~ 0.5 nmol/L(未结合),在人体内转化为~ 100 nmol/L(或40 ng/mL,总浓度)的Ctarget。 阿西替尼抑制小鼠肿瘤生长和血管生成。[2] < br > 阿西替尼抑制小鼠人异种移植肿瘤的生长(表2)。无论初始肿瘤大小、模型类型或植入部位如何,阿西替尼都会产生剂量依赖性生长延迟。重要的是,Axitinib在原位植入肿瘤如M24met(黑色素瘤)、HCT-116(结直肠癌)和SN12C(肾细胞癌)中表现出原发性肿瘤抑制和远处转移控制。MV522肿瘤模型显示出剂量依赖性生长抑制(图4A)。肿瘤生长抑制(TGI)与中心坏死、微血管密度(CD31染色)和Ki-67降低以及肿瘤中caspase-3升高相关(图4B;补充图S3)。无论肿瘤类型和RTK表达如何,在所有肿瘤类型中均观察到类似的效果。总之,阿西替尼治疗在不同类型的肿瘤中产生一致的抗肿瘤功效,这种活性与血管生成和肿瘤增殖的减少以及肿瘤凋亡的增加有关。 ED50和cef的测定。[2] 采用MV522模型测定50%抗肿瘤疗效的有效剂量(ED50)。MV522肿瘤细胞不表达功能性VEGF或PDGF RTKs。此外,肿瘤生长速度适中,是评价阿西替尼抗血管生成相关ED50的理想模型。根据剂量与相应的TGI之间的关系(图4A), ED50被确定为8.8 mg/kg每日两次(补充图S4), 30 mg/kg每日两次的剂量水平对应于该模型中的ED70。通过评估TGI(图4A)与血浆浓度之间的关系,确定50% TGI对应的体内有效浓度(Ceff)范围。根据Cmin(谷浓度),估计未结合Ceff为0.28 nmol/L(或0.11 ng/mL;图4C,左);根据Cave (24 h内平均浓度),计算出的未结合Ceff为0.85 nmol/L (0.33 ng/mL;图4C,右)。因此,Ceff值范围(0.28-0.85 nmol/L)与体内靶调制研究得出的Ctarget值(0.5 nmol/L)一致。 [2] 根据药代动力学、IC50值、Ctarget和Ceff进一步分析剂量与靶标抑制的关系。在一天的大部分时间里,10 mg/kg (ED50剂量)和30 mg/kg (ED70剂量)的血浆浓度均高于和接近Ctarget(对于vegfr)和Ceff(基于tgi)(图4D)。然而,这些剂量下的血浆浓度分别只允许PDGFR-β在细胞IC50上覆盖约5和12小时(体内研究中基于抗PDGFR的Ctarget或Ceff不可用)。基于这一分析,在MV522模型(VEGFR-null, PDGFR-null)中,10 mg/kg的抗肿瘤效果似乎主要来自于阿西替尼/Axitinib对血管VEGFR的抑制。在相同的模型中,注射阿西替尼获得了接近最大的抗肿瘤疗效(80%),这与稳态血浆浓度大于vegfr的细胞IC50而低于PDGFR-β的细胞IC50相关(数据未显示)。 阿西替尼/Axitinib在多种肿瘤模型中增强化疗药物的抗肿瘤疗效。[2] 阿西替尼与多西他赛(在LLC和人类乳腺癌模型中)、卡铂(在人类卵巢癌模型中)或吉西他滨(在人类胰腺癌模型中)联合进行抗肿瘤疗效评估。选择这些模型是因为它们对小鼠的化疗只有低或中等敏感性。 [2] 在LLC模型中,Axitinib(10或30 mg/kg口服,每日2次)联合最大耐受剂量的多西他赛(40 mg/kg每周1次)增强了肿瘤生长延迟,定义为治疗组与对照组相比,到达终点的中位时间(TTE)的增加。TTE(疾病进展的衡量标准)定义为肿瘤大小= 1,500 mm3或动物因肿瘤负担或转移而死亡。多西他赛加10或30 mg/kg阿西替尼组的肿瘤生长延迟分别为54%或100%,而多西他赛单用和30和60 mg/kg阿西替尼组分别为9%、30%和60%(数据未显示)。与单独使用多西紫杉醇相比,多西紫杉醇联合阿西替尼显著延缓了疾病进展(图5A)。在MDA-MB-435/HAL-luc模型中,阿西替尼(30 mg/kg)和多西他赛(5 mg/kg;25%的小鼠最大耐受剂量)产生了强大的肿瘤生长延迟,这表明肿瘤生物发光信号的减少(补充图S5),与单独使用任何一种药物相比,完全应答者的数量增加(数据未显示)。 [2] 在耐吉西他滨的BxPC-3人胰腺癌模型中,研究了阿西替尼联合吉西他滨在不同给药方案下的抗肿瘤疗效(图5B)。在一项研究中,单药吉西他滨(140 mg/kg,每日1、4、7和10天,单周期或三周期治疗)或阿西替尼(30 mg/kg口服,每日2次)延缓肿瘤生长。在接受吉西他滨加阿西替尼治疗的组中,无论吉西他滨疗程的多少,“早期给药”(第1天,第5组)比“晚期给药”(在开始使用吉西他滨后第11天(第6组)或第16天(第7组)开始使用阿西替尼)产生更大的肿瘤生长延迟;在相同的阿西替尼方案下,三个吉西他滨治疗周期(8、9、10组)比一个吉西他滨治疗周期(5、6、7组)产生更大的疗效。两种药物的交替剂量(12组)或早期终止阿西替尼(11组)与共给药和连续每日两次给药阿西替尼相比,肿瘤生长延迟显著降低。 阿西替尼联合贝伐单抗在M24met模型中具有显著的抗转移活性。[2] 研究了阿西替尼增强贝伐单抗在原位植入和自发转移的人黑色素瘤M24met肿瘤模型中的疗效;M24met肿瘤不表达功能性rtk(数据未显示)。最重要的是,在该模型中,贝伐单抗的配体循环人VEGF-A被发现占体内循环VEGF-A总量的95%。 [2] 在本研究中,阿西替尼/Axitinib(60mg /kg口服,每日2次)和贝伐单抗(5mg /kg静脉注射,2xqw)均表现出中等的单药抗淋巴结肿瘤转移活性。两种药物联合使用显著提高了基于淋巴结肿瘤体积减少(图5C)、抗血管生成(补充图S6)和转移性淋巴结肿瘤增殖指数(补充图S7)评估的抗转移疗效。此外,联合治疗通过减少进展时间(TTE)显著延长了动物生存期,单药治疗与对照组相比,TTE均为13天,联合治疗与对照组相比,TTE均为20天(图5D)。正如预期的那样,贝伐珠单抗或贝伐珠单抗联合阿西替尼,而不是阿西替尼单药治疗,显著降低了游离血浆人VEGF-A(数据未显示)。 |
| 酶活实验 |
生成的是过表达全长 VEGFR2、PDGFRβ 的猪主动脉内皮 (PAE) 细胞、Kit 和过表达鼠 VEGFR2 (Flk-1) 或 PDGFRα 的 NIH-3T3 细胞。为了制备 ELISA 捕获板,每孔 100 μL 2.5 μg/mL 抗 VEGFR2 抗体、0.75 μg/mL 抗 PDGFRβ 抗体、0.25 μg/mL 抗 PDGFRα 抗体、0.5 μg/mL 抗 KIT 抗体或 1.20 μg /mL 抗 Flk-1 抗体包被在 96 孔板上。接下来,使用 ELISA 测量 RTK 磷酸化。[2]
|
| 细胞实验 |
将 5 × 104 细胞接种到 96 孔板中,并将细胞培养一整天。将浓度范围为 1 nM 至 10 μM 的阿西替尼添加到细胞中。 72小时后使用MTS四唑底物测量细胞活力,并计算IC50值。
三维球管形成试验[2] 将500个人微血管内皮细胞添加到含有0.24%甲基纤维素的EGM-2培养基中,并将其转移到u底96孔板上过夜,形成球体。收集约50个球体,与含有4%至8%胎牛血清(FBS)的2 mg/mL纤维蛋白原溶液(含或不含化合物)混合,在涂有凝血酶(5000单位/mL)的48孔板中。得到的三维纤维蛋白凝胶覆盖含有4%至8% FBS的EGM-2,并在37℃下孵育。每天在倒置显微镜下观察内皮管的形成。 免疫沉淀和免疫印迹[2] 内皮细胞或肿瘤细胞在1% FBS (HUVEC)或0.1% FBS(肿瘤细胞)存在下饥饿18小时。加入阿西替尼/Axitinib,在1 mmol/L Na3VO4存在下,37°C孵育45 min。在细胞中加入适当的生长因子,5分钟后,用冷PBS冲洗细胞,在裂解缓冲液和蛋白酶抑制剂鸡尾酒中裂解。裂解物与免疫沉淀抗体在4°C下孵育过夜。抗体配合物与蛋白A小珠结合,SDS-PAGE分离上清液。使用Super Signal West硬脑膜试剂盒检测化学发光信号。 |
| 动物实验 |
Mice and Rats: Mice bearing 400–600 mm3 M24met xenograft tumors receive either a single Axitinib dose or 0.5% carboxymethylcellulose/H2O as a control. Samples of blood and tumor tissue are obtained for VEGFR-2 and pharmacokinetic analyses. The Bradford colorimetric assay is used to measure the total protein concentrations in tumor tissues.
Axitinib (30 mg/kg) is injected intraperitoneally twice into six-day-old Sprague-Dawley rats. Retinal tissue is extracted and lysed, animals are sacrificed, and immunoprecipitation and immunoblotting experiments are carried out. The Alpha Imager 8800 is used for densitometry analysis, and ECL-Plus is used for detection. In vivo target modulation [2] VEGFR-2 phosphorylation inhibition in the rat development model. Six-day-old Sprague-Dawley rats were given two i.p. injections of Axitinib. Animals were sacrificed, retinas were collected and lysed, and immunoprecipitation/immunoblotting experiments were done as described above. ECL-Plus was used for detection and densitometry analysis was done using the Alpha Imager 8800. VEGFR-2 phosphorylation inhibition in xenograft tumors. Mice with M24met xenograft tumors (400-600 mm3) were administered with a single dose of Axitinib or the control (0.5% carboxymethylcellulose/H2O). Blood and tumor tissue samples were collected for pharmacokinetic and VEGFR-2 measurements. Total protein concentrations in tumor tissues were determined using the Bradford colorimetric assay. Procedures for immunoprecipitation/immunoblotting and ELISA were as described above. Axitinib pharmacokinetics [2] Plasma concentrations of Axitinib were quantitatively determined by a triple-quadruple mass spectrometer equipped with a high-performance liquid chromatography system (Agilent 1100) using a Phenyl column (Zorbax Eclipse XDB, 5 μm particle size, 50 × 2.1 mm) under isocratic conditions of 60:40 water/acetonitrile containing 0.1% formic acid. Data were collected under multiple reaction monitoring mode of m/z 387.3→356.2 for axitinib and m/z 394.2→360.2 for the internal standard, Axitinib/AG-013736-d7. The method quantified for axitinib over the range of 1 to 1,000 ng/mL in mouse plasma. The area under the plasma concentration-time curve of axitinib was calculated using the linear trapezoidal rule. Skin vascular permeability assay in naive or tumor-bearing mice [2] The assay was done according to Miles and Miles with some modifications. nu/nu mice (n = 5-8) received a single oral dose of Axitinib followed by an injection of 30 μL Evan's blue dye through the tail vein. Thirty minutes later, murine VEGF-A (400 ng in 10 μL PBS) or PBS was injected into the trunk area posterior to the shoulder of the animal. Four hours later, the skin region immediately surrounding the blue color area was dissected and immersed in 1 mL formamide. Evan's blue was extracted by incubating the tissues in formamide at 56°C for 24 h. Vascular permeability was quantified by measuring light absorbance at 620 nm. Mouse xenograft models [2] In general, tumor cells in FBS-depleted medium were implanted s.c. into the right flank region of athymic mice, except for the following: the M24met cells were implanted intradermally in a 50 to 100 μL volume in BALB/C severe combined immunodeficient mice; the procedures for orthotopic implantation of HCT-116-GFP and SN12C-GFP tumors have been described elsewhere; the A375 cells were implanted in the presence of 10% Matrigel; the LLC tumors were inoculated either using the suspension cells or 2 × 2 mm viable tumor fragments via the Trocar needles. Unless otherwise specified, mice were randomized when the average tumor was ∼100 mm3 (9-12 per group). Tumor volumes were measured three times per week by electronic calipers and calculated according to the following equation: 0.5 × [length × (width)2]. Treatment usually lasted for 2 to 4 weeks or until tumors reached 1,500 mm3. The procedure for tumor bioluminescent imaging and quantification using the IVIS Imaging System has been reported elsewhere. Treatments. Axitinib/AG-013736, a receptor kinase inhibitor of VEGFRs and, at higher doses, PDGFRs (IC50 = 0.1 nmol/L for VEGFR-1, 0.2 nmol/L for VEGFR-2, 0.1–0.3 nmol/L for VEGFR-3, and 1.6 nmol/L for PDGFRβ; ref. 18), was provided by Pfizer Global Research and given once daily by gavage in a volume of 0.13 mL. Control animals received 0.5% carboxymethylcellulose drug carrier. Irradiations were done on nonanesthetized mice using a 137Cs source operating at 2.4 Gy/min. Mice were confined to plastic jigs with tumor-bearing legs extended through an opening in the side, allowing local irradiations. Fractionated doses were given in five daily 2 Gy fractions per week (omitting weekends). For combination treatments, radiotherapy was delivered first, and Axitinib/AG-013736 was given within ∼4 h. Mice were sacrificed, and tumors were excised and then quick frozen (using liquid nitrogen) following 1, 2, or 3 weeks of treatment. [1] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
After one 5 mg dose of axitinib, it takes about 2.5 to 4.1 hours to reach maximum plasma concentration. Axitinib is mainly eliminated unchanged in the feces (41%) with 12% of the original dose as unchanged axitinib. There is also 23% eliminated in the urine, most of which are metabolites. The volume of distribution is 160 L. The average clearance of axitinib is 38 L/h. Metabolism / Metabolites Axitinib undergoes mainly hepatic metabolism. CYP3A4 and CYP3A5 are the main hepatic enzymes while CYP1A2, CYP2C19, and UGT1A1 enzymes are secondary. Biological Half-Life Axitinib has a half life of 2.5 to 6.1 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In large clinical trials of axitinib, elevations in serum aminotransferase levels were common, occurring in up to 25% of patients. Values greater than 5 times the upper limit of normal (ULN), however, were uncommon, occurring in 1% to 2% of recipients. Furthermore, no instances of clinically apparent liver injury from axitinib were reported in prelicensure studies or during the more wide scale use since its approval. Nevertheless, periodic monitoring of liver tests during axitinib therapy is recommended. Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of axitinib during breastfeeding. Because axitinib is more than 99% bound to plasma proteins, the amount in milk is likely to be low. The manufacturer recommends that breastfeeding be discontinued during axitinib therapy and for 2 weeks after the final dose of therapy. When axitinib is used in combination with avelumab or pembrolizumab, refer to those LactMed records. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Plasma protein binding for axitinib is high at over 99% with most protein binding to albumin followed by α1-acid glycoprotein. |
| 参考文献 |
|
| 其他信息 |
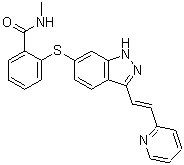
Axitinib is an indazole substituted at position 3 by a 2-(pyridin-2-yl)vinyl group and at position 6 by a 2-(N-methylaminocarboxy)phenylsulfanyl group. Used for the treatment of advanced renal cell carcinoma after failure of a first line systemic treatment. It has a role as an antineoplastic agent, a tyrosine kinase inhibitor and a vascular endothelial growth factor receptor antagonist. It is a member of indazoles, a member of pyridines, an aryl sulfide and a member of benzamides.
Axitinib is a second generation tyrosine kinase inhibitor that works by selectively inhibiting vascular endothelial growth factor receptors (VEGFR-1, VEGFR-2, VEGFR-3). Through this mechanism of action, axitinib blocks angiogenesis, tumour growth and metastases. It is reported to exhibit potency that is 50-450 times higher than that of the first generation VEGFR inhibitors. Axitinib is an indazole derivative. It is most commonly marketed under the name Inlyta® and is available in oral formulations. Axitinib is a Kinase Inhibitor. The mechanism of action of axitinib is as a Receptor Tyrosine Kinase Inhibitor. Axitinib is an oral tyrosine kinase inhibitor selective for vascular endothelial growth factor (VEGF) receptors -1, -2 and -3 that is used in the therapy of advanced renal cell carcinoma. Axitinib therapy is commonly associated with transient elevations in serum aminotransferase that are generally mild and asymptomatic. Axitinib has yet to be linked to instances of clinically apparent acute liver injury. Axitinib is an orally bioavailable tyrosine kinase inhibitor. Axitinib inhibits the proangiogenic cytokines vascular endothelial growth factor (VEGF) and platelet-derived growth factor receptor (PDGF), thereby exerting an anti-angiogenic effect. A benzamide and indazole derivative that acts as a TYROSINE KINASE inhibitor of the VASCULAR ENDOTHELIAL GROWTH FACTOR RECEPTOR. It is used in the treatment of advanced RENAL CELL CARCINOMA. Drug Indication Used in kidney cell cancer and investigated for use/treatment in pancreatic and thyroid cancer. FDA Label Inlyta is indicated for the treatment of adult patients with advanced renal cell carcinoma (RCC) after failure of prior treatment with sunitinib or a cytokine. Mechanism of Action Axitinib selectively blocks the tyrosine kinase receptors VEGFR-1 (vascular endothelial growth factor receptor), VEGFR-2, and VEGFR-3. Pharmacodynamics Axitinib prevents the progression of cancer by inhibiting angiogenesis and blocking tumor growth. In conclusion, the current findings argue against a treatment-induced functional normalization of the tumor vasculature when applying combination therapy. Rather than tightening pericytes, AG-013736 and the combination treatment served instead to loosen pericyte-vessel and pericyte-basement membrane associations in this tumor model. Treatment substantially reduced total and functional vascular densities, but overall tumor hypoxia progressively increased, in contrast to the normalization hypothesis. Despite the reduction in oxygenation, tumor progression was minimal over 3 weeks of combination treatment, most likely due to continued vascular destruction and the prevention of new vessel growth. Further studies are essential to extend these measurements to additional tumor models and to determine whether alternative scheduling may also enhance treatment response. [1] Purpose: Axitinib (AG-013736) is a potent and selective inhibitor of vascular endothelial growth factor (VEGF) receptor tyrosine kinases 1 to 3 that is in clinical development for the treatment of solid tumors. We provide a comprehensive description of its in vitro characteristics and activities, in vivo antiangiogenesis, and antitumor efficacy and translational pharmacology data. Experimental design: The potency, kinase selectivity, pharmacologic activity, and antitumor efficacy of axitinib were assessed in various nonclinical models. Results: Axitinib inhibits cellular autophosphorylation of VEGF receptors (VEGFR) with picomolar IC(50) values. Counterscreening across multiple kinase and protein panels shows it is selective for VEGFRs. Axitinib blocks VEGF-mediated endothelial cell survival, tube formation, and downstream signaling through endothelial nitric oxide synthase, Akt and extracellular signal-regulated kinase. Following twice daily oral administration, axitinib produces consistent and dose-dependent antitumor efficacy that is associated with blocking VEGFR-2 phosphorylation, vascular permeability, angiogenesis, and concomitant induction of tumor cell apoptosis. Axitinib in combination with chemotherapeutic or targeted agents enhances antitumor efficacy in many tumor models compared with single agent alone. Dose scheduling studies in a human pancreatic tumor xenograft model show that simultaneous administration of axitinib and gemcitabine without prolonged dose interruption or truncation of axitinib produces the greatest antitumor efficacy. The efficacious drug concentrations predicted in nonclinical studies are consistent with the range achieved in the clinic. Although axitinib inhibits platelet-derived growth factor receptors and KIT with nanomolar in vitro potencies, based on pharmacokinetic/pharmacodynamic analysis, axitinib acts primarily as a VEGFR tyrosine kinase inhibitor at the current clinical exposure. Conclusions: The selectivity, potency for VEGFRs, and robust nonclinical activity may afford broad opportunities for axitinib to improve cancer therapy. [2] Therapeutic targeting of tumor angiogenesis with VEGF inhibitors results in demonstrable, but transitory efficacy in certain human tumors and mouse models of cancer, limited by unconventional forms of adaptive/evasive resistance. In one such mouse model, potent angiogenesis inhibitors elicit compartmental reorganization of cancer cells around remaining blood vessels. The glucose and lactate transporters GLUT1 and MCT4 are induced in distal hypoxic cells in a HIF1α-dependent fashion, indicative of glycolysis. Tumor cells proximal to blood vessels instead express the lactate transporter MCT1, and p-S6, the latter reflecting mTOR signaling. Normoxic cancer cells import and metabolize lactate, resulting in upregulation of mTOR signaling via glutamine metabolism enhanced by lactate catabolism. Thus, metabolic symbiosis is established in the face of angiogenesis inhibition, whereby hypoxic cancer cells import glucose and export lactate, while normoxic cells import and catabolize lactate. mTOR signaling inhibition disrupts this metabolic symbiosis, associated with upregulation of the glucose transporter GLUT2.[3] |
| 分子式 |
C22H18N4OS
|
|---|---|
| 分子量 |
386.47
|
| 精确质量 |
386.12
|
| 元素分析 |
C, 68.37; H, 4.69; N, 14.50; O, 4.14; S, 8.30
|
| CAS号 |
319460-85-0
|
| 相关CAS号 |
Axitinib-13C,d3;1261432-00-1;Axitinib-d3;1126623-89-9
|
| PubChem CID |
6450551
|
| 外观&性状 |
white to off-white solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
668.9±55.0 °C at 760 mmHg
|
| 熔点 |
213-215ºC
|
| 闪点 |
358.3±31.5 °C
|
| 蒸汽压 |
0.0±2.0 mmHg at 25°C
|
| 折射率 |
1.728
|
| LogP |
4.15
|
| tPSA |
95.97
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
557
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C(C1=C(SC2=CC3=C(C(/C=C/C4=CC=CC=N4)=NN3)C=C2)C=CC=C1)NC
|
| InChi Key |
RITAVMQDGBJQJZ-FMIVXFBMSA-N
|
| InChi Code |
InChI=1S/C22H18N4OS/c1-23-22(27)18-7-2-3-8-21(18)28-16-10-11-17-19(25-26-20(17)14-16)12-9-15-6-4-5-13-24-15/h2-14H,1H3,(H,23,27)(H,25,26)/b12-9+
|
| 化学名 |
N-methyl-2-[[3-[(E)-2-pyridin-2-ylethenyl]-1H-indazol-6-yl]sulfanyl]benzamide
|
| 别名 |
AG 013736; Axitinib; 319460-85-0; AG-13,736; axitinibum; C9LVQ0YUXG; UNII-C9LVQ0YUXG; NSC-757441; N-methyl-2-((3-((1E)-2-(pyridin-2-yl)ethenyl)-1H-indazol-6-yl)sulfanyl)benzamide; AG013736; Axitinib; AG-013736; Brand name: Inlyta
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (5.38 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.08 mg/mL (5.38 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (5.38 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 0.5% CMC: 30mg/mL 配方 5 中的溶解度: 8.33 mg/mL (21.55 mM) in 20% HP-β-CD/10 mM citrate pH 2.0 (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 需要超声波并用H2O调节pH至3。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5875 mL | 12.9376 mL | 25.8752 mL | |
| 5 mM | 0.5175 mL | 2.5875 mL | 5.1750 mL | |
| 10 mM | 0.2588 mL | 1.2938 mL | 2.5875 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Study to Evaluate the Efficacy and Safety of Pembrolizumab (MK-3475) in Combination With Axitinib Versus Sunitinib Monotherapy in Participants With Renal Cell Carcinoma (MK-3475-426/KEYNOTE-426)
CTID: NCT02853331
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-11-18
|
|
|
|