| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Bestatin (Ubenimex) targets p38 mitogen-activated protein kinase (p38 MAPK) (inhibits phosphorylation of p38 MAPK) [2]
Bestatin (Ubenimex) acts on aminopeptidases [4] |
|---|---|
| 体外研究 (In Vitro) |
在 ATRA 敏感的 APL NB4 细胞中,bestatin 促进 ATRA 诱导的分化并防止 ATRA 驱动的 p38 MAPK 激活。 ATRA 抗性 APL MR2 细胞具有贝他汀不可逆的分化阻断作用。当CD13与抗CD13抗体WM-15连接时,p38 MAPK被磷酸化,Bestatin对p38 MAPK磷酸化的抑制作用减弱,并且Bestatin对ATRA诱导NB4细胞分化的增强作用被完全消除[2]。用贝他汀 (600 μM) 处理的细胞会经历细胞周期进程延迟,因为它们的分裂频率和生长速率降低。 Bestatin 抑制 D 的先天多核性和有丝分裂的频率。 discoideum 且不会引起 D 细胞毒性。 0-600 μM 的盘状细胞。在 PsaA-GFP 和 GFP 表达细胞的裂解物中,bestatin 抑制氨肽酶活性分别为对照的 69.39% 和 39.93%[4]。
在急性早幼粒细胞白血病(APL)NB4细胞中,贝他汀(Bestatin,乌苯美司)(50–200 μM)增强全反式维甲酸(ATRA)诱导的细胞分化:流式细胞术显示,200 μM药物+1 μM ATRA处理72小时后,CD11b阳性细胞(分化标志物)比例达65–85%,而ATRA单药组仅为40%;Western blot证实磷酸化p38 MAPK(p-p38)呈剂量依赖性降低[2] - 在盘基网柄菌(Dictyostelium discoideum)细胞中,贝他汀(Bestatin,乌苯美司)(10–100 μM)抑制细胞生长(50 μM处理48小时后生长抑制率为50%)、阻断细胞分裂(100 μM时有丝分裂指数降低60%)、抑制孢子细胞分化(100 μM时孢子形成率降低75%)[4] - 在NB4细胞中,贝他汀(Bestatin,乌苯美司)(100–200 μM)单药对细胞增殖或分化无显著影响,但与ATRA联合使用可协同促进髓系分化[2] |
| 体内研究 (In Vivo) |
与糖尿病媒介物治疗的小鼠相比,Bestatin (20 μM) 可有效降低糖尿病小鼠中的 CD13 表达,并显着抑制 MMP-9 特异性溶胶带密度。 Bestatin 疗法可显着降低糖尿病小鼠中 VEGF 和乙酰肝素酶的表达。玻璃体内贝他汀治疗显着下调糖尿病小鼠视网膜中 HIF-1α 和 VEGF 的表达。此外,玻璃体内注射贝他汀治疗可显着降低糖尿病小鼠视网膜中乙酰肝素酶表达的增加[1]。在对 SRBC 产生抗原增强体液反应之前,Bestatin(10、1 和 0.1mg/kg,腹膜内注射)治疗导致产生溶血性抗 SRBC 抗体 (PFC) 的脾细胞数量增加,并提高 2-ME 抗性血清血凝素滴度(剂量为0.1毫克/千克)。注射环磷酰胺后,每隔一天给小鼠注射贝斯汀(1 和 0.1 mg/kg)5 次,不会改变药物对 PFC 数量的抑制作用,甚至导致剂量下总抗 SRBC 血凝素进一步减少抗原刺激后第 7 天为 1 mg/kg [3]。
在链脲佐菌素(STZ)诱导的糖尿病小鼠模型中,腹腔注射贝他汀(Bestatin,乌苯美司)(10 mg/kg,每日一次,连续4周)保护视网膜结构和功能:视网膜神经节细胞(RGC)凋亡减少(TUNEL阳性细胞较糖尿病对照组降低62%)、视网膜厚度保留(外核层厚度增加35%)、视网膜电图(ERG)b波振幅改善(增加40%)[1] - 在正常BALB/c小鼠中,贝他汀(Bestatin,乌苯美司)(10、20 mg/kg,腹腔注射,每日一次,连续7天)增强对绵羊红细胞(SRBC)的体液免疫应答:抗SRBC抗体滴度较对照组增加50–80%[3] - 在环磷酰胺(CY)诱导的免疫抑制小鼠中,贝他汀(Bestatin,乌苯美司)(20 mg/kg,腹腔注射或口服,每日一次,连续7天)逆转CY介导的免疫抑制:抗SRBC抗体滴度恢复至正常小鼠的70%,脾脏指数(脾脏重量/体重比)较CY处理组增加45%[3] |
| 酶活实验 |
p38 MAPK磷酸化抑制实验:NB4细胞用贝他汀(Bestatin,乌苯美司)(50–200 μM)处理1小时后,加入1 μM ATRA孵育48小时。裂解细胞后,通过Western blot用磷酸化p38 MAPK(p-p38)和总p38 MAPK抗体检测,光密度法量化条带强度,评估p38磷酸化抑制效果[2]
- 氨肽酶活性测定(盘基网柄菌):细胞裂解物与氨酰-p-硝基苯胺底物及系列稀释的贝他汀(Bestatin,乌苯美司)(10–100 μM)在37°C下孵育60分钟。在405 nm波长下检测p-硝基苯胺的释放量,相对于溶媒对照组计算酶活性抑制率[4] |
| 细胞实验 |
NB4细胞分化实验:将NB4细胞接种于6孔板(1×106个细胞/孔),用贝他汀(Bestatin,乌苯美司)(50–200 μM)单药或与1 μM ATRA联合处理72小时。用CD11b抗体染色,流式细胞术分析分化率;Western blot检测p-p38、总p38及CD11b蛋白水平[2]
- 盘基网柄菌生长与分化实验:细胞在含贝他汀(Bestatin,乌苯美司)(10–100 μM)的液体培养基中培养48小时,血细胞计数板计数细胞数量评估生长抑制。分化实验中,细胞接种于无营养琼脂上并加入药物处理,72小时后显微镜下观察并量化孢子形成情况[4] - 视网膜细胞凋亡实验(体外相关):从STZ诱导的糖尿病小鼠中获取视网膜组织外植体,用贝他汀(Bestatin,乌苯美司)(1–10 μM)处理24小时。固定外植体后,TUNEL试剂染色,荧光显微镜下计数凋亡细胞[1] |
| 动物实验 |
4 mg/kg, dis-solved in normal saline; oral gavage
Rats Diabetic retinal protection model: C57BL/6 mice are intraperitoneally injected with STZ to induce diabetes (blood glucose > 16.7 mM). One week later, mice are randomized into diabetic control and treatment groups (n = 8 per group). Bestatin (Ubenimex) is dissolved in physiological saline and administered intraperitoneally at 10 mg/kg once daily for 4 weeks. Retinal tissues are collected for TUNEL staining, histological analysis, and ERG measurement [1] - Humoral immune response model: BALB/c mice are randomly divided into control and treatment groups (n = 6 per group). Bestatin (Ubenimex) (10, 20 mg/kg) is administered intraperitoneally once daily for 7 days; for oral administration, the drug is suspended in 0.5% carboxymethylcellulose. On day 3, mice are immunized with SRBC via intraperitoneal injection. Seven days post-immunization, serum is collected to measure anti-SRBC antibody titer by hemagglutination assay [3] - Immunocompromised mouse model: BALB/c mice are intraperitoneally injected with cyclophosphamide (CY) to induce immune suppression. Twenty-four hours later, mice receive Bestatin (Ubenimex) (20 mg/kg, intraperitoneal or oral) once daily for 7 days, followed by SRBC immunization. Serum antibody titer and spleen index are measured 7 days post-immunization [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
In in vivo studies, Bestatin (Ubenimex) (up to 20 mg/kg, intraperitoneal or oral) in mice causes no significant weight loss, mortality, or abnormal clinical signs during the study period [1][3]
- No significant changes in liver (ALT, AST) or kidney (BUN, creatinine) function markers are observed in STZ-induced diabetic mice treated with Bestatin (Ubenimex) (10 mg/kg, 4 weeks) [1] - In vitro, Bestatin (Ubenimex) shows no cytotoxicity to NB4 cells at concentrations up to 200 μM (cell viability > 90% by MTT assay) [2] |
| 参考文献 |
|
| 其他信息 |
Ubenimex (also known as bestatin) is a competitive protease inhibitor. It is an inhibitor of aminopeptidase B, leukotriene A4 hydrolase, aminopeptidase N. It is being studied for use in the treatment of acute myelocytic leukemia.
Ubenimex has been reported in Streptomyces abikoensis and Streptomyces olivoreticuli with data available. Ubenimex is a microbial metabolite and dipeptide with potential immunomodulatory and antitumor activities. Ubenimex competitively inhibits many aminopeptidases, including B, N and leucine aminopeptidases. Aminopeptidases has been implicated in the process of cell adhesion and invasion of tumor cells. Therefore, inhibiting aminopeptidases may partially attribute to the antitumor effect of ubenimex. This agent also activates T lymphocyte, macrophage and bone marrow stem cell as well as stimulates release of interleukin-1 and -2, thus further enhances its antitumor activity. Drug Indication An adjuvant therapy used for acute and chronic myelonous leukemia, lung cancer and nasopharyngeal cancer. It is also used to treat hypercholesterolaemia. Bestatin (Ubenimex) is a natural aminopeptidase inhibitor with multiple biological activities, including immunomodulatory, anti-tumor, and tissue-protective effects [1][2][3][4] - Its mechanism of enhancing ATRA-induced NB4 cell differentiation involves inhibition of p38 MAPK phosphorylation, which blocks the negative regulatory effect of p38 on myeloid differentiation [2] - In diabetic retina, Bestatin (Ubenimex) exerts protective effects by inhibiting retinal cell apoptosis, possibly via regulating oxidative stress and inflammatory pathways [1] - The immunomodulatory activity of Bestatin (Ubenimex) includes enhancing humoral immunity in normal mice and reversing immune suppression in CY-treated mice, making it a potential adjuvant for immunotherapy [3] - In Dictyostelium discoideum, Bestatin (Ubenimex) inhibits cell growth and differentiation by targeting aminopeptidases involved in cell cycle regulation and developmental signaling [4] |
| 分子式 |
C16H24N2O4
|
|
|---|---|---|
| 分子量 |
308.37
|
|
| 精确质量 |
308.173
|
|
| CAS号 |
58970-76-6
|
|
| 相关CAS号 |
Bestatin hydrochloride;65391-42-6;Bestatin trifluoroacetate;223763-80-2
|
|
| PubChem CID |
72172
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
604.7±55.0 °C at 760 mmHg
|
|
| 熔点 |
245 °C (dec.)(lit.)
|
|
| 闪点 |
319.5±31.5 °C
|
|
| 蒸汽压 |
0.0±1.8 mmHg at 25°C
|
|
| 折射率 |
1.557
|
|
| LogP |
2.64
|
|
| tPSA |
112.65
|
|
| 氢键供体(HBD)数目 |
4
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
8
|
|
| 重原子数目 |
22
|
|
| 分子复杂度/Complexity |
367
|
|
| 定义原子立体中心数目 |
3
|
|
| SMILES |
CC(C)C[C@@H](C(=O)O)NC(=O)[C@H]([C@@H](CC1=CC=CC=C1)N)O
|
|
| InChi Key |
VGGGPCQERPFHOB-RDBSUJKOSA-N
|
|
| InChi Code |
InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1
|
|
| 化学名 |
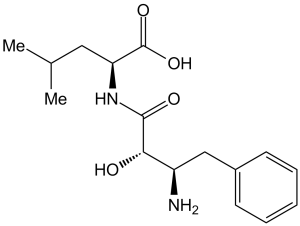
(2S)-2-[[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanoyl]amino]-4-methylpentanoic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 0.83 mg/mL (2.69 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 8.3 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 0.83 mg/mL (2.69 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 8.3 mg/mL 澄清 DMSO 储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 0.83 mg/mL (2.69 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.2429 mL | 16.2143 mL | 32.4286 mL | |
| 5 mM | 0.6486 mL | 3.2429 mL | 6.4857 mL | |
| 10 mM | 0.3243 mL | 1.6214 mL | 3.2429 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。