| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
PD-1/PD-L1 (IC50 = 18 nM); PD-1/PD-L1 (KD = 8 μM)
|
|---|---|
| 体外研究 (In Vitro) |
BMS-202(0-100 μM;4 天;SCC-3 或 Jurkat 细胞)治疗可防止强 PD-L1 阳性 SCC-3 细胞(IC50 为 15 μM)和抗 CD3 抗体激活的 Jurkat 细胞(IC50 10 μM)体外[2]。 BMS-202 特异性诱导 PD-L1 热稳定。 BMS-202 在溶液中引起 PD-L1 二聚化。在同型二聚体的核心,BMS-202 填充了一个大的疏水口袋,促进单体之间的大量额外相互作用。 PD-1/PD-L1 相互作用在生理上由疏水表面介导,BMS-202 与这些表面上的两个 PD-L1 分子相互作用[1]。
|
| 体内研究 (In Vivo) |
在人源化 MHC-dKO NOG 小鼠中,BMS-202(20 mg/kg;腹腔注射;每天;持续 9 天;NOG-dKO 小鼠)治疗与对照组相比表现出明显的抗肿瘤作用[2]。
此外,来自荷瘤裸鼠异种移植的数据支持BMS-202在体内显著抑制U251细胞的生长。综上所述,这些体内和体外数据表明,BMS-202在不影响正常胶质细胞的情况下,显著抑制GBM细胞的生长,为其抗肿瘤应用于GBM提供了一个安全的治疗窗口期。 |
| 酶活实验 |
所有结合研究均在 HTRF 测定缓冲液中进行,该缓冲液由 dPBS 组成,并补充有 0.1%(含 v)牛血清白蛋白和 0.05%(v/v)Tween-20。对于 PD-l-Ig/PD-Ll-His 结合测定,将抑制剂与 PD-Ll-His(最终浓度为 10 nM)在 4 μL 测定缓冲液中预孵育 15 m,然后添加 PD-l- Ig(最终 20 nM)溶于 1 μL 测定缓冲液中,并进一步孵育 15 m。使用来自人类、食蟹猴或小鼠的 PD-L1。 HTRF 检测是使用铕穴酸盐标记的抗 Ig(最终 1 nM)和别藻蓝蛋白 (APC) 标记的抗 His(最终 20 nM)实现的。将抗体在 HTRF 检测缓冲液中稀释,并在结合反应上分配 5 μL。让反应混合物平衡 30 分钟,并使用 En Vision 荧光计获得信号(665 nm/620 nm 比率)。在 PD-1-Ig/PD-L2-His(分别为 20、5 nM)、CD80-His/PD-Ll-Ig(分别为 100、10 nM)和 CD80-His/CTLA4- 之间建立了额外的结合测定。 Ig(分别为 10、5 nM)。
|
| 细胞实验 |
程序性死亡-1/程序性死亡-配体 1 (PD-1/PD-L1) 相互作用在抑制 T 细胞反应中发挥主导作用,特别是在肿瘤微环境中,保护肿瘤细胞免于裂解。据报道,PD-1/PD-L1 抑制剂 2 可阻止 PD-L1 与 PD-1 的相互作用,IC50 值为 18 nM。
|
| 动物实验 |
In an in vivo study using humanized MHC-double knockout (dKO) NOG mice, BMS-202 showed a clear antitumor effect compared with the controls; however, a direct cytotoxic effect was revealed to be involved in the antitumor mechanism, as there was no lymphocyte accumulation in the tumor site. These results suggest that the antitumor effect of BMS-202 might be partly mediated by a direct off-target cytotoxic effect in addition to the immune response-based mechanism. Also, the humanized dKO NOG mouse model used in this study was shown to be a useful tool for the screening of small molecule inhibitors of PD-1/PD-L1 binding that can inhibit tumor growth via an immune-response-mediated mechanism[2].
In vivo therapeutic tumor-bearing xenografts with BMS-202[3] According to our previous studies, 5 × 10~6 U251 cells were mixed with matrigel and subcutaneously injected into the male NOD/SCID nude mice, aged 4–6 weeks (n = 8). When the tumor volume (0.5 × length × width2) reached 100 mm3, the mice were randomly divided into the control group, treated with vehicle, and the BMS-202 group, intraperitoneally injected with 20 mg/kg BMS-202, twice per week. The therapeutic process was stopped until the tumor volumes in the control group reached the ethically approved maximum volume 2000 mm3. |
| 参考文献 |
|
| 其他信息 |
Targeting the PD-1/PD-L1 immunologic checkpoint with monoclonal antibodies has provided unprecedented results in cancer treatment in the recent years. Development of chemical inhibitors for this pathway lags the antibody development because of insufficient structural information. The first nonpeptidic chemical inhibitors that target the PD-1/PD-L1 interaction have only been recently disclosed by Bristol-Myers Squibb. Here, we show that these small-molecule compounds bind directly to PD-L1 and that they potently block PD-1 binding. Structural studies reveal a dimeric protein complex with a single small molecule which stabilizes the dimer thus occluding the PD-1 interaction surface of PD-L1s. The small-molecule interaction "hot spots" on PD-L1 surfaces suggest approaches for the PD-1/PD-L1 antagonist drug discovery.[1]
Recently, the first series of small molecule inhibitors of PD-1/PD-L1 were reported by Bristol-Myers Squibb (BMS), which were developed using a homogeneous time-resolved fluorescence (HTRF)-based screening investigation of the PD-1/PD-L1 interaction. Additional crystallographic and biophysical studies showed that these compounds inhibited the interaction of PD-1/PD-L1 by inducing the dimerization of PD-L1, in which each dimer binds one molecule of the stabilizer at its interface. However, the immunological mechanism of the antitumor effect of these compounds remains to be elucidated. In the present study, we focused on BMS-202 (a representative of the BMS compounds) and investigated its antitumor activity using in vitro and in vivo experiments. BMS-202 inhibited the proliferation of strongly PD-L1-positive SCC-3 cells (IC50 15 μM) and anti-CD3 antibody-activated Jurkat cells (IC50 10 μM) in vitro. Additionally, BMS-202 had no regulatory effect on the PD-1 or PD-L1 expression level on the cell surface of these cells. In an in vivo study using humanized MHC-double knockout (dKO) NOG mice, BMS-202 showed a clear antitumor effect compared with the controls; however, a direct cytotoxic effect was revealed to be involved in the antitumor mechanism, as there was no lymphocyte accumulation in the tumor site. These results suggest that the antitumor effect of BMS-202 might be partly mediated by a direct off-target cytotoxic effect in addition to the immune response-based mechanism. Also, the humanized dKO NOG mouse model used in this study was shown to be a useful tool for the screening of small molecule inhibitors of PD-1/PD-L1 binding that can inhibit tumor growth via an immune-response-mediated mechanism.[2] Recently, crystallographic studies have demonstrated that BMS-202, a small-molecule compound characterized by a methoxy-1-pyridine chemical structure, exhibits a high affinity to PD-L1 dimerization. However, its roles and mechanisms in glioblastoma (GBM) remain unclear. The objective of this study is to investigate the antitumor activity of BMS-202 and its underlying mechanisms in GBM using multi-omics and bioinformatics techniques, along with a majority of in vitro and in vivo experiments, including CCK-8 assays, flow cytometry, co-immunoprecipitation, siRNA transfection, PCR, western blotting, cell migration/invasion assays and xenografts therapeutic assays. Our findings indicate that BMS-202 apparently inhibits the proliferation of GBM cells both in vitro and in vivo. Besides, it functionally blocks cell migration and invasion in vitro. Mechanistically, it reduces the expression of PD-L1 on the surface of GBM cells and interrupts the PD-L1-AKT-BCAT1 axis independent of mTOR signaling. Taken together, we conclude that BMS-202 is a promising therapeutic candidate for patients with GBM by remodeling their cell metabolism regimen, thus leading to better survival.[3] |
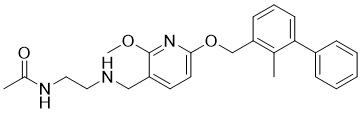
| 分子式 |
C25H29N3O3
|
|
|---|---|---|
| 分子量 |
419.52
|
|
| 精确质量 |
419.22
|
|
| 元素分析 |
C, 71.57; H, 6.97; N, 10.02; O, 11.44
|
|
| CAS号 |
1675203-84-5
|
|
| 相关CAS号 |
N-deacetylated BMS-202;2310135-18-1
|
|
| PubChem CID |
117951478
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.1±0.1 g/cm3
|
|
| 沸点 |
611.4±55.0 °C at 760 mmHg
|
|
| 闪点 |
323.6±31.5 °C
|
|
| 蒸汽压 |
0.0±1.8 mmHg at 25°C
|
|
| 折射率 |
1.575
|
|
| LogP |
3.99
|
|
| tPSA |
72.5Ų
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
10
|
|
| 重原子数目 |
31
|
|
| 分子复杂度/Complexity |
526
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O(C1C([H])=C([H])C(=C(N=1)OC([H])([H])[H])C([H])([H])N([H])C([H])([H])C([H])([H])N([H])C(C([H])([H])[H])=O)C([H])([H])C1C([H])=C([H])C([H])=C(C2C([H])=C([H])C([H])=C([H])C=2[H])C=1C([H])([H])[H]
|
|
| InChi Key |
JEDPSOYOYVELLZ-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C25H29N3O3/c1-18-22(10-7-11-23(18)20-8-5-4-6-9-20)17-31-24-13-12-21(25(28-24)30-3)16-26-14-15-27-19(2)29/h4-13,26H,14-17H2,1-3H3,(H,27,29)
|
|
| 化学名 |
2-[2,6-dichloro-4-(3,5-dimethyl-1,2-oxazol-4-yl)anilino]-N-hydroxybenzamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.96 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.96 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.96 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 4.05 mg/mL (9.65 mM) in 45% PEG300 5% Tween-80 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3837 mL | 11.9184 mL | 23.8368 mL | |
| 5 mM | 0.4767 mL | 2.3837 mL | 4.7674 mL | |
| 10 mM | 0.2384 mL | 1.1918 mL | 2.3837 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
 J Med Chem.2017Jul 13;60(13):5857-5867. |
|---|
|
|
Structural Biology of the Immune Checkpoint Receptor PD-1 and Its Ligands PD-L1/PD-L2.Structure.2017 Aug 1;25(8):1163-1174. |
|---|
New Directions in Designing the Therapeutics Targeting the PD-1/PD-L1 Interaction.Structure.2017 Aug 1;25(8):1163-1174. |
Structural Basis of the PD-1/PD-L1 (PD-L2) Interaction.Structure.2017 Aug 1;25(8):1163-1174. |