| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g | |||
| 100g | |||
| Other Sizes |
| 靶点 |
DNA alkylator
|
|---|---|
| 体外研究 (In Vitro) |
白消安抑制鹅卵石区域形成细胞的频率,但不会显着提高造血干细胞祖细胞和类似细胞的凋亡率。通过不依赖细胞凋亡的机制,白消安抑制 HSC 祖细胞和类似细胞的造血功能。白消安以时间依赖性方式导致骨髓造血细胞衰老,这与 p16Ink4a 和 p19Arf 表达上调有关。[1]正常人二倍体 WI38 成纤维细胞暴露于白消安,这是一种烷化剂,可通过交联 DNA 以及 DNA 和蛋白质来损伤 DNA。该药物通过细胞外信号调节激酶 (Erk) 和 p38 丝裂原激活蛋白激酶 (p38 MAPK) 级联引起衰老,该级联独立于 p53-DNA 损伤途径。白消安会导致 GSH 暂时减少,但 ROS 生成持续增加。[2]通过减少睾丸细胞中 PCNA 的表达,白消安诱导的 Rb 低磷酸化可阻止精原干细胞发生凋亡。 [3]
|
| 体内研究 (In Vivo) |
白消安治疗的小鼠睾丸重量显着下降,细胞凋亡增加。为了最大限度地增加凋亡细胞的数量并最大限度地减少坏死细胞的数量,给予 40 mg/kg 体重的白消安。 [3]使用有限稀释分析,白消安调理和辐射产生的 HSC 检测灵敏度与 NOD/SCID 小鼠相当。 [4]移植白消安的小鼠表现出不完整且缓慢的淋巴移植。接受白消安(20 mg/kg 至 100 mg/kg)的小鼠表现出剂量依赖性的同源淋巴组织重建。 [5]
|
| 酶活实验 |
诱导细胞衰老是正常细胞对DNA损伤剂的常见反应,DNA损伤剂可能导致癌症化疗和电离辐射诱导的正常组织损伤。这种诱导在很大程度上归因于p53的激活。然而,本研究的结果表明,白消安(BU)是一种通过交联DNA、DNA和蛋白质引起DNA损伤的烷化剂,它通过细胞外信号调节激酶(Erk)和p38丝裂原活化蛋白激酶(p38 MAPK)级联诱导正常人二倍体WI38成纤维细胞衰老,与p53 DNA损伤途径无关。WI38细胞衰老的诱导是由细胞内谷胱甘肽(GSH)的短暂耗竭引发的,随后通过烟酰胺腺嘌呤二核苷酸磷酸(NADPH)氧化酶持续增加活性氧(ROS)的产生,从而激活Erk和p38 MAPK通路。用N-乙酰半胱氨酸(NAC)孵育WI38细胞可以补充细胞内GSH,消除ROS产生的增加,改善Erk和p38 MAPK的激活,并减弱BU诱导的衰老。因此,使用有效的抗氧化剂或Erk和p38 MAPK途径的特异性抑制剂抑制衰老诱导具有被开发为改善癌症治疗诱导的正常组织损伤的基于机制的策略的潜力。[2]
雄性生殖细胞凋亡在啮齿动物中得到了广泛的研究。相比之下,人们对发育中的生殖细胞在白消安治疗后对凋亡的敏感性知之甚少。在成年小鼠睾丸中很少观察到生殖细胞的自发凋亡,但在本文所述的实验条件下,白消安治疗的小鼠表现出凋亡的显著增加和睾丸重量的减少。TdT介导的dUTP-X缺口末端标记分析表明,在白消安治疗一周后,凋亡主要局限于精原细胞,对精母细胞的影响较小。凋亡阳性小管百分比和凋亡细胞指数呈时间依赖性增加。在治疗后一周内,精原细胞中观察到了直接效应,在接下来的一周里,精母细胞中也观察到了次要效应。RT-PCR分析显示,精原细胞特异性标记物c-kit和Stra 8的表达降低,但Gli I基因表达保持不变,这表明分化中的A型精原细胞发生了初级凋亡。白消安治疗3周和4周后,RAD51和FasL表达降至几乎检测不到的水平,表明减数分裂精母细胞和减数分裂后细胞分别丢失。尽管p110Rb磷酸化和PCNA表达受到抑制,但白消安处理的睾丸中生殖细胞耗竭的时期与p53或Fas/FasL表达的增加并不一致。这些数据表明,白消安处理的小鼠雄性生殖细胞耗竭的增加是由c-kit/SCF信号传导的丧失介导的,而不是由p53或Fas/FasL依赖的机制介导的。通过调节细胞周期信号,抑制对G1期进展至关重要的E2F依赖性蛋白表达,可以保护精原干细胞免于细胞死亡[3]。 |
| 细胞实验 |
细胞系:WI38 细胞
浓度:120 μM 孵育时间:24 小时 结果:以时间依赖性方式激发中等程度的 p53 激活,但强烈的 Erk、p38 和 JNK 磷酸化。引起 p21 表达立即上调,并在第 11 天消退。 小鼠骨髓(BM)细胞暴露于电离辐射(IR;4 Gy)导致与造血干细胞样细胞(Lin(-)ScaI(+)c-kit(+)细胞)凋亡诱导相关的各种日型鹅卵石区形成细胞的频率受到95%以上的抑制;IR:64.8+/-0.4%,对照组:20.4+/-0.5%;P<0.001)和祖细胞(Lin(-)ScaI(-)c-kit(+)细胞;IR:46.2+/-1.4%,对照组:7.8+/-0.5%;P<0.001)。将小鼠骨髓细胞与白消安(BU;30微M)孵育6小时也抑制了鹅卵石区形成细胞的频率,但未能导致这两种造血细胞的凋亡显著增加。经过5周的长期BM细胞培养后,与对照细胞相比,33%和72%的造血细胞分别存活了IR和BU诱导的损伤,但它们不能形成粒细胞-巨噬细胞集落形成单位。此外,这些存活的细胞表达了更高水平的衰老相关β-半乳糖苷酶p16(Ink4a)和p19(Arf)。这些发现表明,IR主要通过诱导凋亡来抑制造血干细胞样细胞和祖细胞的功能,而BU主要通过诱导早衰来抑制。此外,诱导BM造血细胞过早衰老也有助于IR诱导的造血功能抑制。有趣的是,IR而非BU诱导造血细胞衰老与p53和p21(Cip1/Waf1)表达的升高有关。这表明IR以p53-p21(Cip1/Waf1)依赖的方式诱导造血细胞衰老,而BU对衰老的诱导绕过了p53-p21。[1] |
| 动物实验 |
ICR male mice ranging in age from 8 to 12 weeks (30-40 g)
40 mg/kg (in sesame oil) IP; single dose Human hematopoietic stem cell (HSC) xenotransplantation in NOD/SCID mice requires recipient conditioning, classically achieved by sublethal irradiation. Pretreatment with immunosuppressive and alkylating agents has been reported, but has not been rigorously tested against standard irradiation protocols. Here, we report that treatment of mice with a single dose (35 mg/kg) of Busilvex, an injectable form of busulfan, enables equivalent engraftment compared to 3.5 Gy irradiation. Mice treated with two doses of 25 mg/kg to reduce busulfan toxicity showed increased chimerism. Busulfan conditioning and irradiation resulted in comparable sensitivity of HSC detection as evaluated by limiting dilution analysis.[4] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Completely absorbed from the gastrointestinal tract. Busulfan is a small, highly lipophilic molecule that crosses the blood-brain-barrier. The absolute bioavailability, if a single 2 mg IV bolus injection is given to adult patients, is 80% ± 20%. In children (1.5 - 6 years old), the absolute bioavailability was 68% ± 31%. When a single oral dose is given to patients, the area under the curve (AUC) was 130 ng•hr/mL. The peak plasma concentration when given orally is 30 ng/mL (after dose normalization to 2 mg). It takes 0.9 hours to reach peak plasma concentration after dose normalization to 4 mg. Following administration of 14C- labeled busulfan to humans, approximately 30% of the radioactivity was excreted into the urine over 48 hours; negligible amounts were recovered in feces. Less than 2% of the administered dose is excreted in the urine unchanged within 24 hours. Elimination of busulfan is independent of renal function. 2.52 ml/min/kg [Following an infusion of dose of 0.8 mg/kg every six hours, for a total of 16 doses over four days] The pharmacokinetic disposition of busulfan differs in children versus adults. The mean bioavailability of busulfan is lower in children than in adults; the interindividual variation in bioavailability for oral busulfan is large, particularly in children. In a pharmacokinetic study in children receiving IV busulfan (0.8 or 1 mg/kg based on actual body weight), an estimated volume of distribution of 0.64 L/kg (with an interpatient variability of 11%) was reported. Busulfan, a small and highly lipophilic molecule, easily crosses the blood-brain barrier. Busulfan concentrations in the cerebrospinal fluid (CSF) are approximately equal to concurrent busulfan plasma concentrations. It is not known whether the drug is distributed into milk. For adults receiving busulfan 2, 4, or 6 mg orally as a single dose on consecutive days, the drug exhibits linear kinetics for both the maximum plasma concentration and the area under the concentration-time curve (AUC); a mean peak plasma concentration (normalized to a dose of 2 mg) of about 30 ng/mL was observed. In a study of 12 patients receiving single oral busulfan doses of 4-8 mg, a mean peak plasma concentration (normalized to a dose of 4 mg) of about 68 ng/mL was reported; the time to peak plasma concentration was about 0.9 hours. Busulfan is rapidly and completely absorbed from the GI tract after oral administration of the drug. The effect of food on the bioavailability of busulfan is not known. For more Absorption, Distribution and Excretion (Complete) data for BUSULFAN (11 total), please visit the HSDB record page. Metabolism / Metabolites Busulfan is extensively metabolizes in the hepatic. Busulfan is predominantly metabolized by conjugation with glutathione, both spontaneously and by glutathione S-transferase (GST) catalysis. GSTA1 is the primary GST isoform that facilitates the the metabolism of busulfan. Other GST isoforms that are also involved are GSTM1 and GSTP1. At least 12 metabolites have been identified among which tetrahydrothiophene, tetrahydrothiophene 12-oxide, sulfolane, and 3-hydroxysulfolane were identified. These metabolites do not have cytotoxic activity. After IP injections of 2:3-(14)C-Myleran in the rat, rabbit and mouse, 60% of the urinary radioactivity was found to be in the form of the 3-hydroxy tetrahydrothiophene-1,1-dioxide, a sulphone. It is suggested that in vivo Myleran undergoes a reaction with cysteine or a cysteinyl moiety to form a cyclic sulphonium ion, which in turn undergoes cleavage to the tetrahydrothiophene, oxidation to the 1,1-dioxide and biological hydroxylation to the 3-hydroxy compound. In the rat and mouse 50-60% of a single dose of Myleran-(35)S (10 mg/kg bw) injected intraperitoneally in arachis oil was excreted within 24 to 48 hours, mainly as methane sulphonic acid; a small amount of unchanged Myleran and two unidentified components were present. In the rabbit, methane sulphonic acid was the only metabolite found in the urine. The metabolic fate of busulfan has been studied in rats and humans using (14)C- and (35)S-labeled materials. In humans, as in the rat, almost all of the radioactivity in (35)S-labeled busulfan is excreted in the urine in the form of (35)S-methanesulfonic acid. /It was/ demonstrated that the formation of methanesulfonic acid in vivo in the rat is not due to a simple hydrolysis of busulfan to 1,4-butanediol, since only about 4% of 2,3-(14)C-busulfan was excreted as carbon dioxide, whereas 2,3-(14)C-1,4-butanediol was converted almost exclusively to carbon dioxide. The predominant reaction of busulfan in the rat is the alkylation of sulfhydryl groups (particularly cysteine and cysteine-containing compounds) to produce a cyclic sulfonium compound which is the precursor of the major urinary metabolite of the 4-carbon portion of the molecule, 3-hydroxytetrahydrothiophene-1,1-dioxide. This has been termed a "sulfur-stripping" action of busulfan and it may modify the function of certain sulfur-containing amino acids, polypeptides, and proteins; whether this action makes an important contribution to the cytotoxicity of busulfan is unknown. (14)C busulfan was administered ip (15 mg/kg) to 5 male Sprague-Dawley rats. For 72 hours, the urinary recovery of (14)C was approximately 70% of the total dose, while the fecal excretion was within the range 1.5-2%. The pattern of the urinary metabolites of busulfan was studied by HPLC in combination with radioactivity detection of the pooled urine. At least eight radioactive fractions could be separated. Three major metabolite peaks were identified by GC/MS and NMR spectroscopy: 3-hydroxysulfolane (39% of total urine radioactivity), tetrahydrothiophene 1- oxide (20%), and sulfolane (13%). Busulfan (6%) and tetrahydrofuran (2%) were also identified. A sulfonium ion glutathione conjugate was hypothesised as another metabolite, but was not isolated because it was very unstable. However, another compound was observed. This metabolite co-eluted with the sulfonium ion obtained of the reaction of busulfan with N-acetyl-L-cysteine and produced tetrahydrothiophene when hydrolyzed. Finally, busulfan and the three main metabolites were tested for cytotoxicity on Chinese V79 hamster cells in vitro. Cell toxicity was induced only by busulfan, which indicates that the cytotoxicity in vivo is mediated by the parent compound, as expected. Busulfan is extensively metabolizes in the hepatic. Busulfan is predominantly metabolized by conjugation with glutathione, both spontaneously and by glutathione S-transferase (GST) catalysis. GSTA1 is the primary GST isoform that facilitates the the metabolism of busulfan. Other GST isoforms that are also involved are GSTM1 and GSTP1. At least 12 metabolites have been identified among which tetrahydrothiophene, tetrahydrothiophene 12-oxide, sulfolane, and 3-hydroxysulfolane were identified. These metabolites do not have cytotoxic activity. Route of Elimination: Following administration of 14C- labeled busulfan to humans, approximately 30% of the radioactivity was excreted into the urine over 48 hours; negligible amounts were recovered in feces. Less than 2% of the administered dose is excreted in the urine unchanged within 24 hours. Elimination of busulfan is independent of renal function. Half Life: 2.6 hours Biological Half-Life 2.6 hours The terminal half life /in children from < 6 months up to 17 years old/ ranged from 2.26 to 2.52 hr. The elimination half-life is about 2.6 hours in adults receiving oral busulfan. Following intravenous administration, the mean half life of busulfan ranged from 2.83 hours to 3.90 hours ... . Oral busulfan had a mean half life of 3.87 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Busulfan is an alkylating agent that contains 2 labile methanesulfonate groups attached to opposite ends of a 4-carbon alkyl chain. Once busulfan is hydrolyzed, the methanesulfonate groups are released and carbonium ions are produced. These carbonium ions alkylate DNA, which results in the interference of DNA replication and RNA transcription, ultimately leading to the disruption of nucleic acid function. Specifically, its mechanism of action through alkylation produces guanine-adenine intrastrand crosslinks. This occurs through an SN2 reaction in which the relatively nucleophilic guanine N7 attacks the carbon adjacent to the mesylate leaving group. This kind of damage cannot be repaired by cellular machinery and thus the cell undergoes apoptosis. Interactions Itraconazole reduced busulfan clearance by up to 25% in patients receiving itraconazole compared to patients who did not receive itraconazole. Higher busulfan exposure due to concomitant itraconazole could lead to toxic plasma levels in some patients. Fluconazole had no effect on the clearance of busulfan. Patients treated with concomitant cyclophosphamide and busulfan with phenytoin pretreatment have increased cyclophosphamide and busulfan clearance, which may lead to decreased concentrations of both cyclophosphamide and busulfan. However, busulfan clearance may be reduced in the presence of cyclophosphamide alone, presumably due to competition for glutathione. Busulfan-induced pulmonary toxicity may be additive to the effects produced by other cytotoxic agents. In one study, 12 of approximately 330 patients receiving continuous busulfan and thioguanine therapy for treatment of chronic myelogenous leukemia were found to have portal hypertension and esophageal varices associated with abnormal liver function tests. Subsequent liver biopsies were performed in 4 of these patients, all of which showed evidence of nodular regenerative hyperplasia. Duration of combination therapy prior to the appearance of esophageal varices ranged from 6 to 45 months. With the present analysis of the data, no cases of hepatotoxicity have appeared in the busulfan-alone arm of the study. Long-term continuous therapy with thioguanine and busulfan should be used with caution. Busulfan may cause additive myelosuppression when used with other myelosuppressive drugs. For more Interactions (Complete) data for BUSULFAN (8 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat iv 14-25 mg/kg LD50 Mouse oral 120 mg/kg |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Busulfan is used in combination with cyclophosphamide as a conditioning regimen prior to allogeneic hematopoietic progenitor cell transplantation in patients with chronic myelogenous leukemia (CML) and is designated an orphan drug by the US Food and Drug Administration (FDA) for the treatment of this disease. /VET/ Antineoplastic agent used in adjunct therapy of acute granulocytic leukemias in small animals. Busulfan is an alkylating agent with myeloablative properties and activity against non-dividing marrow cells and, possibly, non-dividing malignant cells. Its use has been well established in the treatment of hematological malignancies, particularly in patients with chronic myeloid leukemia and other myeloproliferative syndromes. Busilvex followed by cyclophosphamide (BuCy4) or melphalan (BuMel) is indicated as conditioning treatment prior to conventional hematopoietic progenitor cell transplantation in pediatric patients. Drug Warnings Myleran is a potent drug. It should not be used unless a diagnosis of chronic myelogenous leukemia has been adequately established and the responsible physician is knowledgeable in assessing response to chemotherapy. Myleran can induce severe bone marrow hypoplasia. Reduce or discontinue the dosage immediately at the first sign of any unusual depression of bone marrow function as reflected by an abnormal decrease in any of the formed elements of the blood. A bone marrow examination should be performed if the bone marrow status is uncertain. Life-threatening hepatic veno-occlusive disease has occurred in patients receiving busulfan (usually in combination with cyclophosphamide or other antineoplastic agents as a component of marrow-ablative therapy prior to bone marrow transplantation). The manufacturer states that a clear causal relationship to busulfan has not been demonstrated. Hepatic veno-occlusive disease diagnosed by clinical examination and laboratory findings occurred in 8% (5/61) of patients receiving IV busulfan in the allogeneic transplant clinical trial and was fatal in 40% (2/5) of cases. Overall mortality from hepatic veno-occlusive disease was 3% for the entire study population. Retrospectively, 3 of the 5 patients diagnosed with hepatic veno-occlusive disease were found to meet the Jones' criteria for this condition. In patients receiving high-dose oral busulfan as a component of a conditioning regimen prior to bone marrow transplant in randomized, controlled studies, the incidence of hepatic veno-occlusive disease was 7.7-12%. Interstitial pneumonitis and pulmonary fibrosis, which rarely were fatal, also have been reported in patients receiving high oral doses of busulfan as a component of a conditioning regimen prior to allogeneic bone marrow transplantation. Nonspecific interstitial fibrosis was diagnosed by lung biopsy in one patient receiving IV busulfan who subsequently died from respiratory failure. In patients receiving oral busulfan, pancytopenia generally occurs with failure to adequately monitor hematologic status and promptly discontinue the drug in response to a large or rapid decrease in leukocyte or platelet counts. Although individual variation in response to the drug does not appear to be an important contributing factor, some patients may be especially sensitive to busulfan and experience abrupt onset of neutropenia or thrombocytopenia. Busulfan-induced pancytopenia may be more prolonged than that induced by other alkylating agents; although recovery may take 1 month to 2 years, the toxicity is potentially reversible and patients should be vigorously supported through any period of severe pancytopenia. Some patients develop bone marrow fibrosis or chronic aplasia which is probably due to busulfan toxicity. Aplastic anemia, sometimes irreversible, has been reported rarely in patients receiving oral busulfan; aplastic anemia usually has occurred following high doses of the drug or long-term administration of conventional doses. For more Drug Warnings (Complete) data for BUSULFAN (42 total), please visit the HSDB record page. Pharmacodynamics Busulfan is an antineoplastic in the class of alkylating agents and is used to treat various forms of cancer. Alkylating agents are so named because of their ability to add alkyl groups to many electronegative groups under conditions present in cells. They stop tumor growth by cross-linking guanine bases in DNA double-helix strands - directly attacking DNA. This makes the strands unable to uncoil and separate. As this is necessary in DNA replication, the cells can no longer divide. In addition, these drugs add methyl or other alkyl groups onto molecules where they do not belong which in turn leads to a miscoding of DNA. Alkylating agents are cell cycle-nonspecific and work by three different mechanisms, all of which achieve the same end result - disruption of DNA function and cell death. Overexpression of MGST2, a glutathione s-transferase, is thought to confer resistance to busulfan. The role of MGST2 in the metabolism of busulfan is unknown however. |
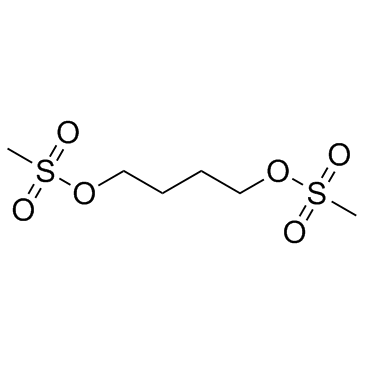
| 分子式 |
C6H14O6S2
|
|---|---|
| 分子量 |
246.3018
|
| 精确质量 |
246.023
|
| 元素分析 |
C, 29.26; H, 5.73; O, 38.98; S, 26.04
|
| CAS号 |
55-98-1
|
| 相关CAS号 |
55-98-1 (Busulfan); 299-75-2 (Treosulfan); 52-24-4 (Thiotepa; Girostan; AI3-24916; NSC-6396)
|
| PubChem CID |
2478
|
| 外观&性状 |
White to khaki solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
464.0±28.0 °C at 760 mmHg
|
| 熔点 |
114-117 °C(lit.)
|
| 闪点 |
234.4±24.0 °C
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
| 折射率 |
1.471
|
| LogP |
-0.52
|
| tPSA |
103.5
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
14
|
| 分子复杂度/Complexity |
294
|
| 定义原子立体中心数目 |
0
|
| SMILES |
S(C([H])([H])[H])(=O)(=O)OC([H])([H])C([H])([H])C([H])([H])C([H])([H])OS(C([H])([H])[H])(=O)=O
|
| InChi Key |
COVZYZSDYWQREU-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C6H14O6S2/c1-13(7,8)11-5-3-4-6-12-14(2,9)10/h3-6H2,1-2H3
|
| 化学名 |
4-methylsulfonyloxybutyl methanesulfonate
|
| 别名 |
Busulfex; Mitosan; Myleran; Mielucin; Misulban; Misulfan; BU; BUS; Myleran; Busulphan; Leucosulfan; Sulphabutin; Busulfex; Myelosan; CB2041; GT41; WR19508
|
| HS Tariff Code |
2905591000
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 63~125 mg/mL (255.8~507.5 mM)
Methanol: ~1 mg/mL (~4.1 mM) H2O: ~1 mg/mL (~4.1 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 6.25 mg/mL (25.38 mM) in 15% Cremophor EL + 85% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (8.44 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (8.44 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.08 mg/mL (8.44 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 配方 5 中的溶解度: 5%DMSO+ 40%PEG300+ 5%Tween 80+ 50%ddH2O: 3.15mg/ml (12.79mM) 配方 6 中的溶解度: 3.12 mg/mL (12.67 mM) in Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.0601 mL | 20.3004 mL | 40.6009 mL | |
| 5 mM | 0.8120 mL | 4.0601 mL | 8.1202 mL | |
| 10 mM | 0.4060 mL | 2.0300 mL | 4.0601 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
CD34+ Enriched Transplants to Treat Myelodysplastic Syndrome
CTID: NCT05617625
Phase: Phase 2 Status: Suspended
Date: 2024-11-25
|
|
|