| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 5g |
|
||
| Other Sizes |

| 靶点 |
5-Lipoxygenase (5-LO); TRPV1; Caffeic acid targets histamine receptor H1 (H1R), transient receptor potential vanilloid 1 (TRPV1), Mas - related G - protein coupled receptor A3 (MrgprA3), transient receptor potential ankyrin 1 (TrpA1), Mas - related G - protein coupled receptor C11 (MrgprC11), and 5 - lipoxygenase (5 - LO).
|
|---|---|
| 体外研究 (In Vitro) |
咖啡酸调节组胺诱导的反应。当光浓度从 0.1 mM 增加到 1 mM 时,咖啡酸的调节作用逐渐增强,模拟正常的剂量调节反应。在用 1 mM 咖啡因处理的 HEK293T-TRPV1 细胞中,辣椒素诱导的反应显着降低。较低剂量的咖啡酸可抑制辣椒素引起的反应。实验表明,咖啡酸可以显着抑制组胺敏感的背根神经节(DRG)神经元。施用咖啡酸 (1 mM) 可使对组胺应用做出反应的 DRG 神经元百分比从 12.5% 降低至 2.1%。 1 mM 咖啡酸可显着抑制 TRPA1 表达细胞中异硫氰酸烯丙酯 (AITC) 诱导的细胞内钙升高。咖啡酸还可能抑制 AITC 诱导的 TRPA1 激活 [1]。
在表达H1R和TRPV1的HEK293T细胞中,咖啡酸可显著阻断组胺诱导的细胞内钙升高。在小鼠背根神经节(DRG)原代培养物中,其也能抑制组胺诱导的细胞内钙升高。在表达MrgprA3和TrpA1的DRG和HEK293T细胞中,咖啡酸可抑制氯喹诱导的反应。此外,它还可降低细胞中由Sligrl - NH2通过MrgprC11诱导的细胞内钙变化,但不能抑制β - 丙氨酸通过MrgprD诱导的反应。 |
| 体内研究 (In Vivo) |
在小鼠模型中,当使用咖啡酸(500 mg/kg)时,观察到组胺诱导的抓挠(30.50±10.87次/1小时,n=6)。此外,虽然似乎有下降趋势(49.40±12.35次/1小时,n=5),但较低剂量咖啡酸(100 mg/kg)在组胺诱导的抓伤中的抗抓伤作用尚未确定具有重要意义。 500 mg/kg 咖啡酸可显着减少氯缓冲液引起的划伤(161.6±31.42 次/1 h,n=5)[1]。在海马区域,咖啡因酸显着降低 5-LO mRNA(P<0.01)。 I/R-咖啡酸组的5-LO蛋白表达显着低于潜在再灌注(I/R)未治疗组(P<0.05或P<0.01)。在比较 I/R-咖啡酸组和 I/R 未治疗组时尤其如此。在低剂量和高剂量咖啡酸组中,整个过程中寻找平台的潜伏期都很显着,并且整个平台潜伏期都在I/R中。 R 组-咖啡酸(50 毫克/千克)。高剂量咖啡酸组海马神经元核固缩明显减少,固缩率为(13.3)±3.0)%,而低剂量组细胞损伤仍然明显(63.6±2.8)%[2] 。
在小鼠抓挠行为实验中,咖啡酸对组胺、氯喹和Sligrl - NH2诱导的抓挠行为有抑制作用。在大鼠全脑缺血再灌注模型中,咖啡酸(10、30、50 mg/kg)可缩短莫里斯水迷宫中的逃避潜伏期,减轻海马神经元损伤,增加神经元数量。同时降低NF - κBp65和5 - LO的表达,减少丙二醛(MDA)含量,增加超氧化物歧化酶(SOD)活性。 |
| 细胞实验 |
瘙痒是一种令人不快的感觉,会引起抓挠的欲望。尽管瘙痒通常被认为是一种微不足道的“令人担忧”的感觉,但它可能会使人衰弱和疲惫,导致生活质量下降。在目前的研究中,研究了咖啡酸是否可以用于缓解各种瘙痒剂引起的瘙痒感的问题,包括组胺、氯喹、SLIGRL-NH2和β-丙氨酸。事实证明,在表达H1R和TRPV1的HEK293T细胞中,组胺诱导的细胞内钙增加被咖啡酸显著阻断,这些分子是组胺诱导的瘙痒在感觉神经元中传播所需的分子。此外,在小鼠背根神经节(DRG)的原代培养物中,咖啡酸抑制组胺诱导的细胞内钙增加。当使用氯喹(一种已知可诱导组胺非依赖性瘙痒的抗疟疾药物)时,还发现咖啡酸抑制表达MRGPRA3和TRPA1的DRG和HEK293T细胞的诱导反应,这是氯喹介导的瘙痒的潜在分子实体。同样,通过PAR2和MRGPRC11的瘙痒诱导剂SLIGRL-NH2引起的细胞内钙变化也被咖啡酸降低。然而,研究发现,咖啡酸不能通过其特异性受体MRGPRD抑制β-丙氨酸诱导的反应[1]。
对于HEK293T细胞,先转染使其表达H1R和TRPV1,然后用不同浓度的咖啡酸处理,最后用组胺刺激,通过钙敏感荧光探针检测细胞内钙浓度。对于DRG原代培养物,在组胺刺激前加入咖啡酸,再以同样方式检测细胞内钙水平。对于表达MrgprA3和TrpA1或MrgprC11的细胞,操作类似,即先加入咖啡酸,再用相应的致痒剂刺激,最后检测细胞内钙变化。 |
| 动物实验 |
Experimental design [2]
Rats were divided into five groups: the sham group (n = 9), I/R non-treated group (n = 9), I/R-caffeic acid group (10 mg · kg−1) (n = 9), I/R-caffeic acid group (30 mg · kg−1) (n = 9) and I/R-caffeic acid group (50 mg · kg−1) (n = 9). In I/R-caffeic acid groups, the rats were administrated caffeic acid at 10, 30, 50 mg · kg−1 (prepared with 0.3% sodium carboxymethyl cellulose) by intraperitoneal injection at 30 min prior to ischemia. The sham group and I/R group were treated with an equal volume of 0.3% sodium carboxymethyl cellulose. Induction of global cerebral I/R model[2] Rats were anesthetized by intraperitoneal injection of chloral hydrate (400 mg/kg), and fixed in a supine position. Global cerebral ischemia was induced as previously described. A midline incision was made in the neck, after that the incision was extended 1 cm to the right. Then both common carotid arteries and the right common jugular vein were exposed carefully by blunt dissection. The distal end of the common jugular vein was ligated following 2 ml heparinized saline (100 mL 0.9% saline containing heparin (250 U)) were perfused. The blood accounting for about 30 percent of the total blood volumes were taken from the right common jugular vein leading to hypotension. Global cerebral ischemia was induced by bilateral clamping of the common carotid arteries combined with hypotension. After ischemia for 20 min, the artery clamps were removed, and the extracted blood was reinfused. Rats in the sham group were subjected to the same operation as above, excepted for the bilateral carotid artery occlusion and hemospasia from the right common jugular vein. In the mouse scratching behavior test, caffeic acid is dissolved in an appropriate solvent and administered to mice by gavage. After a certain period, histamine, chloroquine, or Sligrl - NH2 is injected subcutaneously, and the number of scratching times within a specific time is counted. In the rat global cerebral ischemia - reperfusion model, 45 rats are randomly divided into 5 groups: sham group, ischemia - reperfusion non - treated group, and three caffeic acid treatment groups (10, 30, 50 mg/kg). Caffeic acid is dissolved in an appropriate solvent and administered by intraperitoneal injection 30 minutes before bilateral carotid artery occlusion for 20 min, followed by reperfusion. Morris water maze test is carried out after reperfusion, and then hippocampal tissues are collected for HE staining, SOD activity, MDA content detection, and NF - κBp65 expression detection by immunohistochemistry. |
| 药代性质 (ADME/PK) |
Metabolism / Metabolites
Enzymes involved in its /caffeic acid/ metabolism have not been identified. In the following, caffeic (CA), chlorogenic (CGA), and dihydrocaffeic (DHCA) acids were incubated with hepatocytes and shown to undergo metabolism by cytochrome P450, catechol-O-methyltransferase (COMT), and beta-oxidation enzymes. Ferulic (FA) or dihydroferulic (DHFA) acids, formed as the result of CA- or DHCA-O-methylation by COMT, were also O-demethylated by CYP1A1/2 but not CYP2E1. DHCA or DHFA also underwent side chain dehydrogenation to form CA and FA, respectively, which was prevented by thioglycolic acid, an inhibitor of the beta-oxidation enzyme acyl CoA dehydrogenase. The rates of glutathione conjugate formation catalyzed by NADPH/microsomes (CYP2E1) in decreasing order DHCA>CA>CGA trend which was in reverse order to the rates of their O-methylation by COMT. The CA- and DHCA-o-quinones formed by NADPH/P450 likely inhibited COMT but can readily form glutathione conjugates. CA, DHCA and DHFA were inter-metabolized to each other and to FA by isolated rat hepatocytes whereas FA was metabolized only to CA but not to DHCA or DHFA. CA, DHCA, FA, DHFA and CGA showed a dose-dependent hepatocyte toxicity and the LD(50) (2 h), determined were in decreasing order of effectiveness DHCA>CA>DHFA>CGA>FA. In summary, evidence has been provided that O-methylation, GSH conjugation, hydrogenation and dehydrogenation are involved in the hepatic metabolism of CA and DHCA. The O-methylation pathway for CA and DHCA is a detoxification route whereas o-quinones formation catalyzed by P450 is the toxification route. In rats, chlorogenic acid is hydrolysed in the stomach and intestine to caffeic and quinic acids. A number of metabolites have been identified. Glucuronides of meta-coumaric acid and meta-hydroxyhippuric acid appear to be the main metabolites in humans. After oral administration of caffeic acid to human volunteers, O-methylated derivatives (ferulic, dihydroferulic and vanillic acids) were excreted rapidly in the urine, while the meta-hydroxyphenyl derivatives appeared later. The dehydroxylation reactions were ascribed to the action of intestinal bacteria. Caffeic Acid has known human metabolites that include (2S,3S,4S,5R)-6-[4-[(E)-2-carboxyethenyl]-2-hydroxyphenoxy]-3,4,5-trihydroxyoxane-2-carboxylic acid and (2S,3S,4S,5R)-6-[5-[(E)-2-carboxyethenyl]-2-hydroxyphenoxy]-3,4,5-trihydroxyoxane-2-carboxylic acid. |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
Caffeic acid enhanced the uptake of radioactive glucose into C2C12 cells in a concentration-dependent manner. Similar effect of phenylephrine on the uptake of radioactive glucose was also observed in C2C12 cells. Prazosin attenuated the action of caffeic acid in a way parallel to the blockade of phenylephrine. Female ICR/Ha mice, nine weeks of age, were fed a diet containing 0.06 mmol/g (10 g/kg of diet) caffeic acid (purity, 99%). From experimental day 8, the mice were also given 1 mg benzo(a)pyrene by gavage twice a week for four weeks. The diet containing caffeic acid was removed three days after the last benzo(a)pyrene treatment. Mice were killed at 211 days of age. In the 17 effective mice, the number of forestomach tumors (> or = 1 mm)/mouse (histology unspecified) was significantly decreased by caffeic acid (p < 0.05) (3.1 versus 5.0 tumors/mouse among 38 mice treated with benzo(a)pyrene alone). Antidote and Emergency Treatment Basic treatment: Establish a patent airway. Suction if necessary. Watch for signs of respiratory insufficiency and assist respirations if necessary. Administer oxygen by nonrebreather mask at 10 to 15 L/min. Monitor for pulmonary edema and treat if necessary ... . Monitor for shock and treat if necessary ... . For eye contamination, flush eyes immediately with water. Irrigate each eye continuously with normal saline during transport ... . Do not use emetics. For ingestion, rinse mouth and administer 5 ml/kg up to 200 ml of water for dilution if the patient can swallow, has a strong gag reflex, and does not drool. Activated charcoal is not effective ... . Do not attempt to neutralize because of exothermic reaction. Cover skin burns with dry, sterile dressings after decontamination ... . /Organic acids and related compounds/ Bronstein, A.C., P.L. Currance; Emergency Care for Hazardous Materials Exposure. 2nd ed. St. Louis, MO. Mosby Lifeline. 1994., p. 152-3 Advanced treatment: Consider orotracheal or nasotracheal intubation for airway control in the patient who is unconscious, has severe pulmonary edema, or is in respiratory arrest. Early intubation, at the first sign of upper airway obstruction, may be necessary. Positive pressure ventilation techniques with a bag valve mask device may be beneficial. Monitor cardiac rhythm and treat arrhythmias as necessary ... . Start an IV with D5W /SRP: "To keep open", minimal flow rate/. Use lactated Ringer's if signs of hypovolemia are present. Watch for signs of fluid overload. Consider drug therapy for pulmonary edema ... . For hypotension with signs of hypovolemia, administer fluid cautiously. Consider vasopressors if patient is hypotensive with a normal fluid volume. Watch for signs of fluid overload ... . Use proparacaine hydrochloride to assist eye irrigation ... . /Organic acids and related compounds/ Bronstein, A.C., P.L. Currance; Emergency Care for Hazardous Materials Exposure. 2nd ed. St. Louis, MO. Mosby Lifeline. 1994., p. 153 Medical Surveillance PRECAUTIONS FOR "CARCINOGENS": Whenever medical surveillance is indicated, in particular when exposure to a carcinogen has occurred, ad hoc decisions should be taken concerning ... /cytogenetic and/or other/ tests that might become useful or mandatory. /Chemical Carcinogens/ Non-Human Toxicity Excerpts /LABORATORY ANIMALS: Subchronic or Prechronic Exposure/ Five male Fischer 344 rats, six weeks of age, were given 20 g/kg of diet caffeic acid (purity, > 98%) in basal diet for four weeks. The forestomachs of all treated animals showed epithelial hyperplasia. No hyperplasia was detected in the five untreated controls. IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: /LABORATORY ANIMALS: Subchronic or Prechronic Exposure/ A group of 15 male Syrian golden hamsters, seven weeks of age, was fed a diet containing 1% (10 g/kg diet) caffeic acid (purity, > 98%) for 20 weeks. The dose level of 1% was selected as one-fourth the LD50 in rats. The stomachs and urinary bladders were processed for histopathological and autoradiographic examinations. Mild epithelial hyperplasia of the forestomach was noted in 14/15 treated animals (severe in one) and in 7/15 untreated animals (p < 0.001). Assessment of (3)H-thymidine incorporation revealed an increase in the number of labelled cells in the forestomach and pyloric region of the glandular stomach as compared with untreated rats, but this was not statistically significant. IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: /LABORATORY ANIMALS: Chronic Exposure or Carcinogenicity/ The carcinogenic potential of caffeic acid was investigated in both sexes of F344 rats and C57BL/6N x C3H/HeN F1 mice. After groups of 30 animals received diet containing 0 and 2.0% caffeic acid for 104 weeks in rats or 96 weeks in mice, detailed histopathological examination revealed induction of forestomach squamous cell papillomas or carcinomas in rats at high incidence (77% for males; 80% for females) and in mice at low incidence (13% for males; 3% for females). Invasion to the abdominal cavity of these squamous cell carcinomas was observed in three rats and two mice. In addition, renal tubular cell hyperplasias and adenomas, clearly related to toxic lesions, were found in treated rats at high incidence for males (73 and 13%) and low incidence for females (20 and 0%). In mice, renal tubular cell hyperplasias and tumors also occurred in treated females (97 and 28%), and at a lower incidence in treated males (27 and 3%). No toxic renal injuries were apparent in mice. Alveolar type II cell tumors also developed in treated male mice (27%) with statistical significance. Thus, the current investigation showed caffeic acid to exert carcinogenic activity for the forestomach squamous cell epithelium in both sexes of F344 rats and C57BL/6N x C3H/HeN F1 mice, for the renal tubular cell in male rats and female mice, and for the alveolar type II cell in male mice. PMID:1913684 /LABORATORY ANIMALS: Chronic Exposure or Carcinogenicity/ Two groups of 20 female Sprague-Dawley rats, 50 days of age, received 25 mg/kg body weight 7,12-dimethylbenz(a)anthracene in 0.5 ml sesame oil by gavage. One week later, the animals were fed a diet containing 0.5% (5 g/kg of diet) caffeic acid (purity, > 99%) for 51 weeks. The mammary glands, ear ducts, stomach, liver and kidneys were examined. The incidence of papillomas of the forestomach was significantly increased (6/19) in animals treated with 7,12-dimethylbenz(a)anthracene alone (0/19; p < 0.01). No other significant increase in tumor incidence was found. IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V56 124 (1993) |
| 参考文献 | |
| 其他信息 |
Caffeic Acid can cause cancer according to The World Health Organization's International Agency for Research on Cancer (IARC).
3,4-dihydroxycinnamic acid appears as yellow prisms or plates (from chloroform or ligroin) or pale yellow granules. Alkaline solutions turn from yellow to orange. (NTP, 1992) Caffeic acid is a hydroxycinnamic acid that is cinnamic acid in which the phenyl ring is substituted by hydroxy groups at positions 3 and 4. It exists in cis and trans forms; the latter is the more common. It has a role as a plant metabolite, an EC 1.13.11.33 (arachidonate 15-lipoxygenase) inhibitor, an EC 2.5.1.18 (glutathione transferase) inhibitor, an EC 1.13.11.34 (arachidonate 5-lipoxygenase) inhibitor, an antioxidant and an EC 3.5.1.98 (histone deacetylase) inhibitor. It is a hydroxycinnamic acid and a member of catechols. Caffeic Acid has been reported in Salvia miltiorrhiza, Salvia plebeia, and other organisms with data available. Caffeic Acid is an orally bioavailable, hydroxycinnamic acid derivative and polyphenol, with potential anti-oxidant, anti-inflammatory, and antineoplastic activities. Upon administration, caffeic acid acts as an antioxidant and prevents oxidative stress, thereby preventing DNA damage induced by free radicals. Caffeic acid targets and inhibits the histone demethylase (HDM) oncoprotein gene amplified in squamous cell carcinoma 1 (GASC1; JMJD2C; KDM4C) and inhibits cancer cell proliferation. GASC1, a member of the KDM4 subgroup of Jumonji (Jmj) domain-containing proteins, demethylates trimethylated lysine 9 and lysine 36 on histone H3 (H3K9 and H3K36), and plays a key role in tumor cell development. Caffeic acid is a metabolite found in or produced by Saccharomyces cerevisiae. See also: Black Cohosh (part of); Comfrey Root (part of); Arctium lappa Root (part of) ... View More ... Mechanism of Action Caffeic acid phenethyl ester (CAPE) was synthesized from caffeic acid and phenethyl alcohol (ratio 1:5) at room temperature with dicyclohexyl carbodiimide (DCC) as a condensing reagent. The yield was about 38%. CAPE was found to arrest the growth of human leukemia HL-60 cells. It also inhibits DNA, RNA and protein synthesis in HL-60 cells with IC50 of 1.0 M, 5.0 M and 1.5 M, respectively. In an attempt to understand the antihyperglycemic action of caffeic acid, the myoblast C2C12 cells were employed to investigate the glucose uptake in the present study. Caffeic acid enhanced the uptake of radioactive glucose into C2C12 cells in a concentration-dependent manner. Similar effect of phenylephrine on the uptake of radioactive glucose was also observed in C2C12 cells. Prazosin attenuated the action of caffeic acid in a way parallel to the blockade of phenylephrine. Effect of caffeic acid on alpha1-adrenoceptors was further supported by the displacement of [3H]prazosin binding in C2C12 cells. Moreover, the glucose uptake-increasing action of phenylephrine in C2C12 cells was inhibited by the antagonists of alpha1A-adrenoceptors, both tamsulosin and WB 4101, but not by the antagonist of alpha1B-adrenoceptors, chlorethylclonidine (CEC). The presence of alpha1A-adrenoceptors in C2C12 cells can thus be considered. Similar inhibition of the action of caffeic acid was also obtained in C2C12 cells co-incubating these antagonists. An activation of alpha1A-adrenoceptors seems responsible for the action of caffeic acid in C2C12 cells. In the presence of U73312, the specific inhibitor of phospholipase C, caffeic acid-stimulated uptake of radioactive glucose into C2C12 cells was reduced in a concentration-dependent manner and it was not affected by U73343, the negative control of U73312. Moreover, chelerythrine and GF 109203X diminished the action of caffeic acid at concentrations sufficient to inhibit protein kinase C. Therefore, the obtained data suggest that an activation of alpha1A-adrenoceptors in C2C12 cells by caffeic acid may increase the glucose uptake via phospholipase C-protein kinase C pathway. Caffeic acid (CA, 3,4-dihydroxycinnamic acid), at 2% in the diet, had been shown to be carcinogenic in forestomach and kidney of F344 rats and B6C3F1 mice. Based on its occurrence in coffee and numerous foods and using a linear interpolation for cancer incidence between dose 0 and 2%, the cancer risk in humans would be considerable. In both target organs, tumor formation was preceded by hyperplasia, which could represent the main mechanism of carcinogenic action. The dose-response relationship for this effect was investigated in male F344 rats after 4-week feeding with CA at different dietary concentrations (0, 0.05, 0.14, 0.40, and 1.64%). Cells in S-phase of DNA replication were visualized by immunohistochemical analysis of incorporated 5-bromo-2'-deoxyuridine (BrdU), 2 hr after intraperitoneal injection. In the forestomach, both the total number of epithelial cells per millimeter section length and the unit length labeling index of BrdU-positive cells (ULLI) were increased, about 2.5-fold, at 0.40 and 1.64%. The lowest concentration (0.05%) had no effect. At 0.14%, both variables were decreased by about one-third. In the kidney, the labeling index in proximal tubular cells also indicated a J-shaped (or U-shaped) dose response with a 1.8-fold increase at 1.64%. In the glandular stomach and in the liver, which are not target organs, no dose-related effect was seen. The data show a good correlation between the organ specificity for cancer induction and stimulation of cell division. With respect to the dose-response relationship and the corresponding extrapolation of the animal tumor data to a human cancer risk, a linear extrapolation appears not to be appropriate. Caffeic acid is a phenolic compound widely distributed in medicinal plants. It has anti - pruritic effects by inhibiting multiple itch transmission pathways, and has a protective effect on global cerebral ischemia - reperfusion injury in rats, which may be related to the inhibition of 5 - LO and the regulation of oxidative stress - related indicators. |
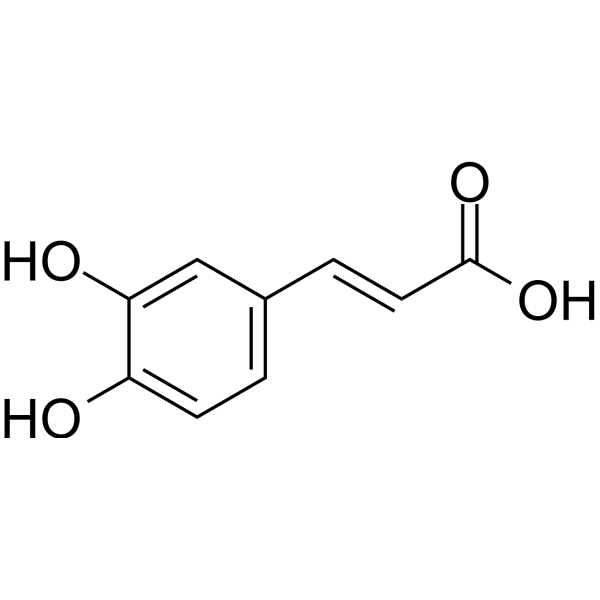
| 分子式 |
C9H8O4
|
|---|---|
| 分子量 |
180.1574
|
| 精确质量 |
180.042
|
| CAS号 |
331-39-5
|
| 相关CAS号 |
trans-Caffeic acid;501-16-6;Caffeic acid phenethyl ester;104594-70-9;Caffeic acid-13C3;1185245-82-2
|
| PubChem CID |
689043
|
| 外观&性状 |
Off-white to light yellow solid
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
416.8±35.0 °C at 760 mmHg
|
| 熔点 |
211-213 °C (dec.)(lit.)
|
| 闪点 |
220.0±22.4 °C
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
| 折射率 |
1.707
|
| LogP |
1.42
|
| tPSA |
77.76
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
13
|
| 分子复杂度/Complexity |
212
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C(O)/C=C/C1=CC=C(O)C(O)=C1
|
| InChi Key |
QAIPRVGONGVQAS-DUXPYHPUSA-N
|
| InChi Code |
InChI=1S/C9H8O4/c10-7-3-1-6(5-8(7)11)2-4-9(12)13/h1-5,10-11H,(H,12,13)/b4-2+
|
| 化学名 |
(E)-3-(3,4-dihydroxyphenyl)prop-2-enoic acid
|
| 别名 |
caffeic acid; 3,4-Dihydroxycinnamic acid; 331-39-5; 3,4-Dihydroxybenzeneacrylic acid; (E)-3-(3,4-dihydroxyphenyl)prop-2-enoic acid; Cinnamic acid, 3,4-dihydroxy-; 3-(3,4-Dihydroxyphenyl)-2-propenoic acid; 2-Propenoic acid, 3-(3,4-dihydroxyphenyl)-;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~555.06 mM)
H2O : < 0.1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (13.88 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (13.88 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (11.55 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.5506 mL | 27.7531 mL | 55.5062 mL | |
| 5 mM | 1.1101 mL | 5.5506 mL | 11.1012 mL | |
| 10 mM | 0.5551 mL | 2.7753 mL | 5.5506 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。