| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
hGHS-R1a ( Ki = 7 nM )
|
|---|---|
| 体外研究 (In Vitro) |
Capromorelin 可刺激大鼠垂体细胞培养物中的 GH 释放,EC50 为 3 nM[2]。
通过在HEK293细胞中使用[125I]-ghrelin作为放射性配体过表达克隆的人GHS-R1a(hGHS-R1a)的竞争性结合试验测量结合亲和力Capromorelin的效力比GHS-R1a的内源性配体ghrelin低17倍(见表1)。Capromorelin的结合活性显示出立体特异性,吡唑啉酮哌啶杂环的3aR异构体优于3aS异构体。PP基团上N-甲基基团的去除导致活性损失两倍。N-乙基PP衍生物5b在测定中与胃饥饿素等效。[1] 尽管表现出30倍的结合亲和力,但Capromorelin和类似物5b和5c以相似的效力刺激GH释放(见表1)。Capromorelin(5a)的3aS非对映异构体显示出明显较弱的活性,EC50值大于1μM。有趣的是,化合物3的3aR-和3aS-非对映异构体(PP二肽的结构前体)都能有效刺激GH分泌(EC50s<25 nM),尽管3aS-非对映异构体稍受青睐。9除5a外,GHS的结合亲和力并不能预测细胞测定中的活性,这可能是因为全细胞测定中蛋白质结合的潜在混杂效应。 |
| 体内研究 (In Vivo) |
在静脉注射后,在麻醉的大鼠模型中测量了PP二肽类似物的体内GH活性Capromorelin和化合物5b和5c在单次1mg/kg剂量后刺激GH分泌,尽管Capromerelin和5c的平均GH峰高明显高于5b的平均GH峰值高度(数据未显示)Capromorelin和5c在模型中显示出类似的剂量-反应关系,ED50值小于0.05 mg/kg iv。N-乙基PP衍生物5b的体内活性较弱归因于其亲脂性增加,这可能减少了血浆室中能够与GHS-R1a相互作用的未结合药物的量。[1]
在狗模型中检查了Capromorelin的口服活性,发现该模型可以预测人类的GHS活性。在单次口服1 mg/kg剂量后,Capromorelin迅速增加了血浆GH水平,最大峰高为73 ng/mL。在低至0.05 mg/kg的剂量下观察到明显的GHS活性。当以1 mg/kg口服给药5天后,Capromolelin在研究的第一天和最后一天刺激了GH分泌;然而,第5天的给药后GH反应有所减弱。[1] 接受卡普莫瑞林 (30 mg/mL) 治疗的狗的食物消耗量明显高于接受安慰剂治疗的狗。卡普莫瑞林组的所有狗体重增加了 0.52 公斤,比安慰剂组多[1]。 Capromorelin 在啮齿类动物模型中表现出增强的肠道吸收,并表现出优异的药代动力学特性,包括在两种动物物种中的高生物利用度 [F(大鼠)= 65%,F(狗)= 44%][2]。 Capromorelin 可刺激麻醉大鼠模型中的 GH 释放,静脉注射 ED50 为 0.05 mg/kg [2]。 |
| 酶活实验 |
结合分析[1]
用质粒pcDNA3.1neo中稳定转染人GHS-R1a受体cDNA的HEK293细胞(ATCC)制备膜。用预浸在0.3%聚乙烯亚胺中的GF/C过滤器以96孔格式进行竞争性放射性配体结合分析。在室温下使用50 pM[125I]-ghrelin和每孔1μg膜在50 mM HEPES、pH 7.4、10 mM MgCl2、0.2%牛血清白蛋白和以下蛋白酶抑制剂中进行1小时的两次检测:100μg/mL杆菌肽、100μg/mL苯甲脒、5μg/mL抑肽酶、5μg/mL亮肽。收集膜并用含有50mM HEPES、pH 7.4和10mM MgCl2的冰冷洗涤缓冲液洗涤三次。使用Prism by Graphpad™测定IC50和Ki值。[125I]-ghrelin在表达人GHS受体的膜上的Kd计算为0.2 nM。在未转染的HEK293细胞膜中未观察到可检测的结合(数据未显示)。 将质粒pcDNA3.1neo中的人GHS-R1a受体cDNA转染至HEK293细胞(ATCC)中以产生膜。竞争性放射性配体结合测定使用已预先浸泡在 0.3% 聚乙烯亚胺中的 GF/C 过滤器以 96 孔形式进行。测试在室温下重复进行一小时,使用 50 pM [125I]-ghrelin 和每孔 1 μg 膜,溶于 50 mM HEPES、pH 7.4、10 mM MgCl2< /sub>、0.2% 牛血清白蛋白,以及随后的蛋白酶抑制剂:100 μg/mL 苯甲脒、100 μg/mL 杆菌肽、5 μg/mL 抑肽酶和 5 μg/mL 亮肽素。收获膜后,在 pH 7.4、50 mM HEPES 和 10 mM MgCl2 的冰冷缓冲液中冲洗 3 次。 GraphpadTM 的 Prism 用于计算 IC50 和 Ki 值。经测定,[125I]-ghrelin 在表达人 GHS 受体的膜上的 Kd 为 0.2 nM。 |
| 细胞实验 |
体外功能活性(大鼠垂体细胞培养中GH的释放)[1]
通过酶促分离6周龄雄性Wistar大鼠的垂体前腺建立原代垂体细胞培养物。将细胞悬浮在Dulbecco改良的Eagle培养基(DMEM,4.5 g/L葡萄糖,补充1 mM丙酮酸钠,1%MEM非必需氨基酸,10%热灭活马血清,2.5%胎牛血清加抗生素)中,以每孔1×105个细胞的速度接种在24孔组织培养板上,并在37°C下在加湿的5%CO2/95%空气培养箱中培养。电镀后3-4天进行激素释放。将细胞培养物漂洗两次,然后在37°C下在释放培养基(DMEM,含25 mM HEPES缓冲液,pH 7.4和5 mg/mL牛血清白蛋白)中平衡30分钟。将该培养基吸出,并用含有测试试剂的预热释放培养基替换。在37°C下孵育15分钟后,取出培养基并测定GH。结果以四个孔的平均值±SEM表示。除非另有说明,否则采用非配对学生的双侧t检验对各组进行比较。数据被报告为产生半最大响应(EC50)所需的浓度。MK-0677用作对照板的参考标准。 |
| 动物实验 |
Pharmacokinetics of Capromorelin in rats[1]
Eight adult female Sprague–Dawley rats were prepared for use by surgical implantation of a cannula in the femoral vein while under methoxyflurane anesthesia the day before study initiation. Study rats were fasted overnight prior to dosing, allowed free access to water and housed in standard polycarbonate rodent cages. The rodents were allowed access to food 4 h post-dose. The dose (intravenous and oral) was prepared as a 0.5 mg/mL base equivalent (0.5 mgA/kg) solution of Capromorelin in deionized water. Four Sprague–Dawley rats (weighing 0.280–0.305 kg) received an iv dose of 1 mgA/kg of Capromorelin. The dose was administered via the femoral vein catheter followed by a 1 mL infusion of normal saline to rinse the catheter. Blood samples (0.5 mL) were taken from the femoral vein catheter pre-dose, 0.083, 0.17, 0.25, 0.5, 0.75, 1, 2, 4, 6 and 8 h post-dose and transferred into heparinized microtainers. The blood volume withdrawn was replaced with normal saline. Plasma was harvested from the centrifuged microtainers and stored frozen at −70 °C in 500 μL polypropylene microcentrifuge tubes. Four Sprague–Dawley rats (weighing 0.275–0.290 kg) received an oral dose of 1 mgA/kg Capromorelin. The dose was administered via an 18-gauge, 3 in., curved gavage needle. Blood samples (0.5 mL) were taken from the femoral vein catheter pre-dose, 0.083, 0.17, 0.25, 0.5, 0.75, 1, 2, 4, 6 and 8 h post-dose and transferred into heparinized microtainers. The blood volume withdrawn was replaced with normal saline. Plasma was harvested from the centrifuged microtainers and stored frozen at −70 °C in 500 μL polypropylene microcentrifuge tubes. Pharmacokinetics of Capromorelin in dogs[1] Four adult beagle dogs (2 males and 2 females weighing 8.9–12.9 kg) were fasted overnight prior to dosing but were allowed free access to water. The dogs were allowed access to food 4 h post-dose. The iv dose was prepared as a 10 mgA/mL solution of Capromorelin in deionized water and filter sterilized. The oral dose was prepared as a 2 mgA/mL solution of Capromorelin in deionized water. Four beagle dogs (weighing 8.9–12.9 kg) received an oral dose of 1 mgA/kg of Capromorelin. The dose was administered via an oral gavage tube. Blood samples (2 mL) were taken from the jugular vein pre-dose, 0.17, 0.33, 0.5, 0.75, 1, 2, 4, 6 and 8 h post-dose. Blood samples were collected directly into heparinized vacutainers. Plasma was harvested from the centrifuged vacutainers and stored frozen at −70 °C in 500 μL polypropylene microcentrifuge tubes. Following a 2 day wash-out period, the same four beagle dogs received an iv dose of 1 mgA/kg of Capromorelin. The dose was administered via the cephalic vein. Blood samples (2 mL) were taken from the jugular vein pre-dose, 0.083, 0.17, 0.33, 0.5, 0.75, 1, 2, 4, 6 and 8 h post-dose. Blood samples were collected directly into heparinized vacutainers. Plasma was harvested from the centrifuged vacutainers and stored frozen at −70 °C in 500 μL polypropylene microcentrifuge tubes. The study evaluated a flavored oral solution containing 30 mg/mL of capromorelin in comparison to a matched placebo-flavored oral solution treatment that was given for four days and contained all the formulation's ingredients but no capromorelin. Two groups of dogs are randomly assigned; Group 1 receives a placebo (0.1 mL/kg), while Group 2 receives 3.0 mg/kg. Each day, at around nine in the morning, both groups receive the same treatment. Day 0 is the first day of medication. A syringe inserted into the mouth's corner is used to administer both the test medication and the placebo. When calculating doses, the Day 0 weight is utilized. |
| 药代性质 (ADME/PK) |
Pharmacokinetic properties of two PP dipeptide GHSs, Capromorelin and 5c, were measured in female Sprague–Dawley rats. For Capromorelin, plasma clearance (CL), volume of distribution (Vd) and plasma elimination half-life (t1/2) following a 1 mgA/kg iv dose were 34±5 mL/min/kg, 1.7 L/kg and 0.79±0.21 h respectively. Capromorelin was rapidly absorbed after a 1 mgA/kg oral (po) dose, reaching maximum systemic concentrations (Cmax) of 329±158 ng/mL in 0.25 h. The unbound plasma free fraction was high (Fu=27%), possibly explaining the robust GHS activity in the in vivo anesthesized rat model. Oral bioavailability was 65%, far greater than the reported bioavailabilities of other peptidyl or peptidomimetic GHSs in the literature. Compound 5c was detected as a circulating metabolite, though exposure generally represented less than 10% of parent drug. Pharmacokinetic values for 5c were similar to those for Capromorelin. Following a 1 mgA/kg iv dose, systemic clearance was high and volume of distribution was moderate, resulting in a short half-life (CL=56.7 mL/min/kg; Vd=2.6 L/kg; t1/2=0.83 h). Oral bioavailability was low, most likely due to the combination of incomplete intestinal absorption and high CL (F=12%).[1]
As the only PP GHS with superior bioavailability and good absorption properties in a rat model, Capromorelin was selected for pharmacokinetic evaluation in the dog. After a 1 mgA/kg iv dose, the plasma clearance of the compound was 19±5 mL/min/kg, the volume of distribution was 2.0±0.4 L/kg and the elimination half-life was 1.3 h. Mean values for Cmax and Tmax following a 1 mgA/kg po dose were 180±66 ng/mL and 1 h. The plasma free fraction of drug was 51% and the bioavailability was 44%. Compound 5c was also identified as a circulating plasma metabolite.[1] Pharmacokinetic characterization of Capromorelin in rats and dogs revealed short plasma elimination half-lives. In dogs, the short half-life was attributed to a moderate volume of distribution and a moderate-to-high clearance due in part to de-methylation of the PP ring and oxidation of both the benzyl group in the (d)-O-Bn-Ser moiety and a methyl group in the Aib moiety (data not shown). The compound did not undergo proteolytic degradation in plasma. The des-methyl metabolite 5c exhibited a similarly short half-life, but because of its lower rat bioavailability, it did not offer any advantages over Capromorelin. A short half-life was considered pharmacologically desirable for stimulating GH secretion (rather than increasing IGF-1 levels) and preventing attenuation of the post-dose GH response during repeat administration.[1] |
| 参考文献 |
|
| 其他信息 |
Capromorelin is under investigation in clinical trial NCT00527046 (Effects Of An Oral Growth Hormone Secretagogue In Older Functionally Limited Adults).
See also: Capromorelin Tartrate (has salt form). Novel pyrazolinone-piperidine dipeptide derivatives were synthesized and evaluated as growth hormone secretagogues (GHSs). Two analogues, capromorelin (5, CP-424391-18, hGHS-R1a K(i)=7 nM, rat pituicyte EC(50)=3 nM) and the des-methyl analogue 5c (hGHS-R1a K(i)=17 nM, rat pituicyte EC(50)=3 nM), increased plasma GH levels in an anesthesized rat model, with ED(50) values less than 0.05 mg/kg iv. Capromorelin showed enhanced intestinal absorption in rodent models and exhibited superior pharmacokinetic properties, including high bioavailabilities in two animal species [F(rat)=65%, F(dog)=44%]. This short-duration GHS was orally active in canine models and was selected as a development candidate for the treatment of musculoskeletal frailty in elderly adults.[1] |
| 分子式 |
C32H41N5O10
|
|---|---|
| 分子量 |
655.705
|
| 精确质量 |
655.29
|
| 元素分析 |
C, 58.62; H, 6.30; N, 10.68; O, 24.40
|
| CAS号 |
193273-69-7
|
| 相关CAS号 |
193273-66-4; 193273-69-7 (tartrate)
|
| PubChem CID |
9852610
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
1.388
|
| tPSA |
218.81
|
| 氢键供体(HBD)数目 |
6
|
| 氢键受体(HBA)数目 |
12
|
| 可旋转键数目(RBC) |
12
|
| 重原子数目 |
47
|
| 分子复杂度/Complexity |
1010
|
| 定义原子立体中心数目 |
4
|
| SMILES |
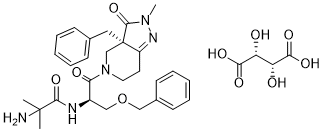
O=C1[C@@]2(CC3C=CC=CC=3)C(CCN(C([C@@H](COCC3C=CC=CC=3)NC(C(C)(C)N)=O)=O)C2)=NN1C.O[C@@H](C(=O)O)[C@H](C(=O)O)O
|
| InChi Key |
MJGRJCMGMFLOET-MYPSAZMDSA-N
|
| InChi Code |
InChI=1S/C28H35N5O4.C4H6O6/c1-27(2,29)25(35)30-22(18-37-17-21-12-8-5-9-13-21)24(34)33-15-14-23-28(19-33,26(36)32(3)31-23)16-20-10-6-4-7-11-20;5-1(3(7)8)2(6)4(9)10/h4-13,22H,14-19,29H2,1-3H3,(H,30,35);1-2,5-6H,(H,7,8)(H,9,10)/t22-,28-;1-,2-/m11/s1
|
| 化学名 |
N-[(2R)-1-[(3aR)-3a-benzyl-2-methyl-3-oxo-6,7-dihydro-4H-pyrazolo[4,3-c]pyridin-5-yl]-1-oxo-3-phenylmethoxypropan-2-yl]-2-amino-2-methylpropanamide;(2R,3R)-2,3-dihydroxybutanedioic acid
|
| 别名 |
Capromorelin tartrate; CP424,391; CP-424391; CP-424,391; CP 424,391;CP 424391; CP424391; CP-424,391-18
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~100 mg/mL (~152.5 mM)
H2O: ~100 mg/mL (~152.5 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (3.81 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (3.81 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (3.81 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 50 mg/mL (76.25 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5251 mL | 7.6253 mL | 15.2506 mL | |
| 5 mM | 0.3050 mL | 1.5251 mL | 3.0501 mL | |
| 10 mM | 0.1525 mL | 0.7625 mL | 1.5251 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|