| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Akt2 (IC50 = 6 nM); p70S6K (IC50 = 120 nM); PKA (IC50 = 168 nM); Autophagy; Apoptosis
|
|---|---|
| 体外研究 (In Vitro) |
CCT128930 对于人胶质母细胞瘤细胞 (U87MG) 的 GI50 值为 6.3 M,对于人前列腺癌细胞 (LNCaP) 的 GI50 值为 0.35 M,对于人前列腺癌细胞 (PC3) 的 GI50 值为 1.9 M,所有这些都是 PTEN 缺陷的人肿瘤细胞系。 1]。当暴露于化合物 CCT128930(0.1-60 M;1 小时)时,人胶质母细胞瘤细胞系 U87MG 在丝氨酸 473 处表现出 AKT 磷酸化的初始诱导,最高可达 20 M,随后在较高浓度[1] 时磷酸化降低。由于相应总蛋白和 GAPDH 水平相对恒定,CCT128930 在 10 M 时抑制下游靶标 pSer235/236 S6RP,在 5 M 时抑制 AKT 的直接底物(Ser9 GSK3、pThr246 PRAS40 和 pT24 FOXO1/p32 FOXO3a) [1]。
CCT128930是一种新型的ATP竞争性AKT抑制剂,是通过基于片段和结构的方法发现的。它是一种强效、先进的吡咯并嘧啶先导化合物,通过靶向单个氨基酸差异,对AKT的选择性高于PKACCT128930在体外多个肿瘤细胞系中表现出明显的抗增殖活性,并抑制了一系列AKT底物的磷酸化,这与AKT抑制一致CCT128930在PTEN缺失的U87MG人胶质母细胞瘤细胞中引起G(1)阻滞,与AKT通路阻断一致。[1] PI3K/Akt/mTOR通路在肿瘤进展和抗癌药物耐药性中起着重要作用。本研究的目的是确定新型Akt小分子抑制剂CCT128930对HepG2肝癌癌症细胞的抗肿瘤作用。我们的结果表明,在低浓度下,CCT128930增加但不抑制HepG2和A549细胞中Akt的磷酸化。CCT128930通过下调cyclinD1和Cdc25A,上调p21、p27和p53,诱导细胞周期阻滞在G1期,从而抑制细胞增殖。更高剂量(20μM)的CCT128930通过激活caspase-3、caspase-9和PARP引发细胞凋亡。CCT128930处理增加了HepG2细胞中ERK和JNK的磷酸化。CCT128930激活了HepG2细胞的DNA损伤反应,其特征是H2AX、ATM(共济失调毛细血管扩张症突变)、Chk1和Chk2的磷酸化。当暴露于更高浓度的CCT128930时,HepG2细胞表现出自噬,同时LC3-II和Beclin-1水平升高。使用氯喹阻断自噬会放大CCT128930诱导的凋亡细胞死亡和H2AX的磷酸化。本研究的结果推进了我们目前对CCT128930在癌症细胞中抗癌机制的理解[2]。 |
| 体内研究 (In Vivo) |
腹膜内注射 25 mg/kg 的 CCT128930 在已建立的 PTEN 缺失 U87MG 人胶质母细胞瘤异种移植物中显示出显着的抗肿瘤作用,第 12 天处理:对照 (T/C) 比率为 48%。40 mg/kg 的 CCT128930 也发挥有效的抗肿瘤作用在 HER2 阳性、PIK3CA 突变型 BT474 人乳腺癌异种移植物中发挥作用,第 22 天完全生长停滞,T/C 比为 29%。静脉注射时,CCT128930 达到 6.4 M 的血浆峰值浓度,然后被快速清除分布容积高、半衰期短、曲线下面积 (AUC0-) 为 4.6 M h。 CCT128930 的腹膜内给药导致血浆药物浓度峰值为 1.3 M,相关 AUC0- 为 1.3 Mh。 CCT128930口服时,血浆峰浓度仅为0.43 M,AUC0-低至0.4 Mh。[1]
CCT128930的体内药效学活性[1] 在体外证明了CCT128930对多种AKT生物标志物的浓度依赖性和时间依赖性抑制作用,并在体内证明了肿瘤暴露的有希望水平,然后在携带U87MG人胶质母细胞瘤肿瘤的同一小鼠中评估了该化合物的药效学作用,用于药代动力学研究(图4B)。图4C和D总结了CCT128930治疗(50 mg/kg i.p.×4天)对最后一次给药后2小时和6小时收获的U87MG异种移植物中几种AKT生物标志物的影响(见图4B)CCT128930在2小时和6小时的时间点均导致AKT上Ser473磷酸化显著增加(分别为P<0.001和P<0.01),与体外生物标志物结果一致(比较图3和图2)。此外,在2小时和6小时的时间点,Ser9 GSK3β(分别为P<0.001和P<0.01)、Ser235/236 S6RP(分别为P<0.05和P<0.01)和Thr246 PRAS40(分别为<0.05和P<0.05)的磷酸化明显降低,蛋白质的总形式保持相对恒定(图4C和D)。这些观察结果与体内U87MG肿瘤中CCT128930对AKT活性的抑制一致。 CCT128930的抗肿瘤活性[1] 接下来,在两种分子相关的人肿瘤异种移植物模型中评估了CCT128930的抗肿瘤活性。图5A显示,在已建立的PTEN缺失的U87MG人胶质母细胞瘤异种移植物中,25 mg/kg腹腔注射(7天×5次)的CCT128930具有明显的抗肿瘤作用,在第12天的治疗:对照(T/C)比率为48%。这种疗法没有减肥的效果。用CCT128930(40mg/kg bid×7天5次)治疗HER2-阳性、PIK3CA-突变BT474人癌症异种移植物也具有显著的抗肿瘤作用,在第22天完全停止生长,T/C比率为29%。该方案与最小的体重减轻有关,在治疗的第15天,最低体重仅为初始体重的94.8%。这些结果清楚地表明,在与PI3K通路激活分子相关的两种人类肿瘤异种移植物模型中,CCT128930作为单一药物具有抗肿瘤活性。 |
| 酶活实验 |
使用 10 μM CCT128930,ATP 浓度相当于每种酶的 Km,对 50 种不同的人类激酶进行分析。
|
| 细胞实验 |
将细胞接种到 96 孔板中并贴壁 36 小时,以确保处理前呈指数生长。使用 96 小时 SRB 测定法测定体外抗增殖活性。 TCA 固定的细胞用溶解在 1% 乙酸中的 0.4%(重量/体积)SRB 染色 30 分钟。染色期结束时,除去 SRB,并用 1% 乙酸快速冲洗培养物四次以除去未结合的染料。将乙酸从烧杯中直接倒入培养孔中。该程序允许快速进行冲洗,从而不会发生蛋白质结合染料的解吸。通过在水槽上猛烈地弹动板来去除残留的洗涤溶液,这确保了漂洗溶液的完全去除。由于 96 孔板中的毛细管作用很强,当板简单倒置时,仅靠重力排水通常无法去除冲洗溶液。冲洗后,将培养物风干直至看不到残留水分。使用 10 mM 无缓冲 Tris 碱 (pH 10.5) 在旋转摇床上溶解结合染料 5 分钟。 OD 在 UVmax 微量滴定板读数器或 Beckman DU-70 分光光度计中读取。为了获得最大灵敏度,OD 在 564 nm 处测量。然而,由于读数与染料浓度仅低于 1.8 OD 单位呈线性关系,因此通常使用次优波长,以便实验中的所有样品都保持在线性 OD 范围内。对于大多数细胞系,大约 490-530 nm 的波长非常适合此目的。
细胞活力测定和细胞集落形成测定[2] 如其他地方报道的那样,MTT法用于检测细胞存活率。简而言之,将细胞铺在96孔板上24小时。然后取出培养基,用不同浓度的CCT128930处理细胞。加入20μL MTT(5mg/mL)4小时。去除上清液后,加入150μL DMSO以溶解甲酰胺晶体,在490 nm处检测吸光度。对于细胞集落形成试验,以每孔1500个细胞(6孔细胞培养板)接种HepG2细胞,并用不同浓度的CCT128930处理2周。将细胞固定在1%戊二醛中,并用0.5%结晶紫染色。在倒置显微镜下计数>30个细胞的菌落。 细胞周期分析[2] 细胞用CCT128930处理24小时。处理结束时,收集细胞,用冰冷的磷酸盐缓冲盐水(PBS)洗涤,并在4°C的70%冷乙醇中固定过夜。用PBS洗涤后,用RNase A消化细胞并用PI染色。使用带有CellQuest软件的FACSCalibur流式细胞术分析样品的DNA含量。 |
| 动物实验 |
6-8 weeks old female CrTacNCr-Fox1nu mice [1]
25 mg/kg (U87MG human glioblastoma xenografts) or 40 mg/kg (BT474 human breast cancer xenografts) i.p. daily for 5 days (U87MG human glioblastoma xenografts); i.p. twice daily for 5 days (BT474 human breast cancer xenografts) In vivo Human Tumor Xenograft Studies [1] PTEN-null U87MG human glioblastoma cells (2×106) were injected subcutaneously (s.c.) in the right flank of 6-8 weeks old female CrTacNCr-Fox1nu mice. For HER2-positive, PIK3CA-mutant BT474 human breast cancer xenografts, cells (5 × 106) were administered s.c. in medium supplemented with matrigel (1:1) into the mammary fat pads of female mice implanted s.c. with estradiol pellets (0.025 mg, 90 day release #NE-121) 3 days previously. Animals were randomized and treatment was started with vehicle or CCT128930 when established tumors were ~100 mm3 in mean volume. Control mice received vehicle only (10% DMSO, 5% Tween 20, 85% saline) and treated mice received 50 mg/kg CCT128930 intraperitoneally (i.p.) daily for 5 days (U87MG human glioblastoma xenografts) or 40 mg/kg CCT128930 i.p. twice daily for 5 days (BT474 human breast cancer xenografts). Tumor size and body weight were monitored three times a week. Tumor size was evaluated by measurement of 2 orthogonal diameters with calipers and volume was calculated from the formula: V= 4/3π[(d1+d2)/4]3. At the end of the study, tumors were excised and weighed. To assess the pharmacokinetic and pharmacodynamic profiles of CCT128930, a single dose of compound (50 mg/kg i.p.) was administered to mice bearing U87MG human glioblastoma xenografts. Plasma and tumor samples were harvested at 2 and 6 hours following dosing. Mice were bled by cardiac puncture and plasma samples were collected and frozen at −20°C until analysis. Tumors were dissected, divided into two approximately equal pieces and snap frozen in liquid nitrogen until analysis. For pharmacodynamic studies, tumors were homogenized using a buffer containing 50 mmol/L Tris (pH 7.4), 1 mmol/L NaCl, 1 mmol/L EDTA, 1% Triton X-100, 1 mmol/L NaF, 1 mmol/L NaVO4, 5 μmol/L Fenvalerate, 5 μmol/L Vbphen, 10 mg/mL TLCK, 1× Complete inhibitor tablet per 10-mL buffer, protease inhibitor cocktail, and phosphatase inhibitor 1 and 2. Protein content was measured using Bradford reagent and samples were analyzed using immunoblotting as described above. Pharmacokinetic Analysis [1] Concentrations of CCT128930 in biological samples were determined using LC-MS. Drug was extracted using methanol and chromatography carried out on a Synergi-Polar RP column (5.0 cm × 4.6 mm ID, 4 µm particle size) using a Waters 600MS pump and a 717 autosampler with a gradient mobile phase of 0.1% formic acid/methanol at 0.6ml/min over 12 minutes. Detection was by LC-MS using a TSQ700 triple quadrapole in which the analyte was ionized by electrospray interface in positive mode to monitor the transition [M+H]+ 342.8 to 146.6.4. The spray voltage was optimized to 5.5Kv and capillary temperature to 260ºC. The assay was linear over the range 10 – 10,000 nM CCT128930. PRAS40 Immunofluorescence Studies [1] U87MG human glioblastoma cells were plated at 5 × 104 cells/well on cover slips in 24-well plates for 36 hours, before being treated with increasing concentrations of CCT128930 for 24 hours. Cells were fixed in 3.8% formaldehyde and permeabilized with 0.01% Triton X-100. BALB/c mice were treated with 50 mg/kg CCT128930 i.p. daily for 4 days, before whiskers were plucked and immediately fixed by immersing, root first, in 10% formal saline for 30 minutes before storing in 4% saline at 4°C. Hair follicles from healthy volunteers were collected and processed as described above. The whisker and hair follicles underwent antigen retrieval with a citrate buffer prior to analysis. The cells, whisker and hair follicles were stained with anti-phospho-Thr246 PRAS40 and anti-PRAS40 antibodies (1:200 for cells; 1:50 for whisker and hair follicles), then visualized with 1:1000 AlexaFluor® 488 goat anti-rabbit IgG antibody. Nuclei were counterstained with 1:10,000 TOPRO-3. The cells or follicles were mounted using Vectashield and images were visualized and captured using a Leica SP1 confocal laser scanning fluorescence microscope All images were taken with the same detector settings based on the respective vehicle-treated control sample. Optical magnification was 250, with an additional 2-fold software (digital) amplification, giving a total magnification of 500. For follicles, images were taken from the middle of the bulb along the z-axis and included the entire cross-section of the bulb. Fluorescence intensity in individual cells was quantified using the INCell Investigator Developer Toolbox v1.6 software. TOPRO-3 was used to identify all nuclei in the image. The areas around the boundary of the nuclei were then expanded to identify cytoplasmic regions. The nuclear and cytoplasmic segmentations were linked together, so that only cytoplasmic areas of the image within cells with nuclei were quantified. The mean fluorescence intensity per cell was then reported from both the pThr246 PRAS40 and total PRAS40 images, respectively. |
| 药代性质 (ADME/PK) |
Pharmacokinetics of CCT128930 [1]
The pharmacokinetics of CCT128930 were determined to establish if therapeutically active drug concentrations could be established in vivo. The pharmacokinetics of CCT128930 after a single dose of 25 mg/kg are illustrated in Figure 4A and summarized in Supplementary Table 1. Following i.v. administration, CCT128930 reached a peak concentration of 6.4 µM in plasma and was eliminated with a relatively short half-life, high volume of distribution and rapid clearance, giving an AUC0-∞ of 4.6 µMh. Following i.p. administration, the peak plasma drug concentration was 4-fold lower and the plasma clearance was similar to that observed i.v‥ The corresponding AUC0-∞ was 1.3 µMh, giving an i.p. bioavailability of 29%. Oral CCT128930 administration gave a comparable pharmacokinetic profile to other routes, but the peak plasma concentration was only 0.43 µM, with a plasma clearance comparable to that observed iv suggesting no first pass metabolism (Supplementary Table 1). This resulted in a correspondingly low AUC and an oral bioavailability of only 8.5%. More importantly, following i.p. administration, peak tumor CCT128930 concentrations were 6-fold higher than the corresponding plasma value at 8 µM and there was evidence of drug retention, as shown by the 2-fold longer half-life and 6-fold slower apparent clearance. This resulted in much higher tumor drug exposure relative to plasma with an AUC0-∞ of 25.8 µMh. Tumor:plasma drug concentrations did not reach steady-state but varied from 4:1 at 30 minutes to 163:1 at 6 hours, confirming tissue drug retention. Assuming linear kinetics, these data supported the use of higher doses and repeat administration to achieve potentially therapeutic tumor drug concentrations in vivo. Figure 4B shows that following CCT128930 administration at 50 mg/kg i.p. per day for 4 days, drug concentrations in U87MG human glioblastoma tumors were consistently much greater than the corresponding plasma concentrations with tumor:plasma ratios of 27:1 and 42:1 at 2 and 6 hours following the last dose, respectively. Moreover, U87MG tumor drug concentrations greatly exceeded the CCT128930 GI50 for at least 6 hours following the final dose and were consistently 5-fold higher than the concentrations required for in vitro biomarker modulation (Figure 2). Metabolism studies revealed that only 0.23% of an administered dose (25 mg/kg i.p.) was recovered unaltered in urine after 24 hours (data not shown). The above results showed that pharmacologically active concentrations of CCT128930 were achieved in tumor tissue at well-tolerated doses. |
| 参考文献 |
|
| 其他信息 |
AKT is frequently deregulated in cancer, making it an attractive anticancer drug target. CCT128930 is a novel ATP-competitive AKT inhibitor discovered using fragment- and structure-based approaches. It is a potent, advanced lead pyrrolopyrimidine compound exhibiting selectivity for AKT over PKA, achieved by targeting a single amino acid difference. CCT128930 exhibited marked antiproliferative activity and inhibited the phosphorylation of a range of AKT substrates in multiple tumor cell lines in vitro, consistent with AKT inhibition. CCT128930 caused a G(1) arrest in PTEN-null U87MG human glioblastoma cells, consistent with AKT pathway blockade. Pharmacokinetic studies established that potentially active concentrations of CCT128930 could be achieved in human tumor xenografts. Furthermore, CCT128930 also blocked the phosphorylation of several downstream AKT biomarkers in U87MG tumor xenografts, indicating AKT inhibition in vivo. Antitumor activity was observed with CCT128930 in U87MG and HER2-positive, PIK3CA-mutant BT474 human breast cancer xenografts, consistent with its pharmacokinetic and pharmacodynamic properties. A quantitative immunofluorescence assay to measure the phosphorylation and total protein expression of the AKT substrate PRAS40 in hair follicles is presented. Significant decreases in pThr246 PRAS40 occurred in CCT128930-treated mouse whisker follicles in vivo and human hair follicles treated ex vivo, with minimal changes in total PRAS40. In conclusion, CCT128930 is a novel, selective, and potent AKT inhibitor that blocks AKT activity in vitro and in vivo and induces marked antitumor responses. We have also developed a novel biomarker assay for the inhibition of AKT in human hair follicles, which is currently being used in clinical trials. [1]
In conclusion, we have evaluated the pharmacological and therapeutic properties of the interesting and novel AKT inhibitor CCT128930. This is a leading example of the pyrrolopyrimidines series, with which we obtained selectivity for AKT over PKA by targeting a single amino acid difference, which we were the first to show. We have demonstrated that CCT128930 suppresses the phosphorylation of downstream markers of AKT in vitro and in vivo and exhibits promising single agent antitumor activity in molecularly relevant human cancer models with appropriate pharmacokinetic and pharmacodynamic properties. We have also used this AKT inhibitor to develop a novel assay to quantify pharmacodynamic biomarker changes in a normal tissue following PI3K-AKT pathway blockade, and this assay may have broader applications in clinical trials of other related pathway inhibitors. [1] Recent reports suggest that autophagy plays an important role in determining cell fate although apoptosis has been widely studied as a cellular response to DNA damage. Autophagy is a ubiquitous highly conserved pathway in eukaryotic cells that takes place as a response to a variety of conditions, such as nutrient deprivation, growth factors withdrawal, and oxidative stress. Conversion of LC3 from cytosolic form (LC3-I) to the proteolytic and lipidated form (LC3-II) is a characteristic hallmark of autophagy. We found that CCT128930 could induce autophagy evidenced by increased levels of LC3-II in HepG2 cells. It will also be interesting to investigate whether inhibitors of autophagy can enhance the anticancer activity of CCT128930. We noted that combination of CCT128930 and CQ led to the desired effects on apoptosis in HepG2 cells. Targeting the autophagy pathway may be a promising therapeutic strategy to enhance CCT128930 lethality via apoptosis for HCC treatment. In conclusion, our results suggest that CCT128930 inhibits cancer cell growth. CCT128930 can induce cell cycle arresting, DNA damage and autophagy in a dose dependent manner. Treatment of the cells with CCT128930 significantly enhanced phosphorylation of ERK1/2 and JNK1/2. Inhibition of autophagy with a lysosomal protease inhibitor CQ potentiates CCT128930's apoptosis-inducing activity and anticancer activity in cancer cells. [2] |
| 分子式 |
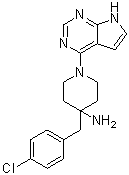
C18H20CLN5
|
|---|---|
| 分子量 |
341.84
|
| 精确质量 |
341.14
|
| 元素分析 |
C, 63.24; H, 5.90; Cl, 10.37; N, 20.49
|
| CAS号 |
885499-61-6
|
| 相关CAS号 |
CCT128930 hydrochloride;2453324-32-6
|
| PubChem CID |
17751819
|
| 外观&性状 |
Off-white to yellow solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
547.9±50.0 °C at 760 mmHg
|
| 闪点 |
285.2±30.1 °C
|
| 蒸汽压 |
0.0±1.5 mmHg at 25°C
|
| 折射率 |
1.680
|
| LogP |
2.93
|
| tPSA |
70.83
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
418
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC1C=CC(CC2(CCN(C3C4=C(NC=C4)N=CN=3)CC2)N)=CC=1
|
| InChi Key |
RZIDZIGAXXNODG-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C18H20ClN5/c19-14-3-1-13(2-4-14)11-18(20)6-9-24(10-7-18)17-15-5-8-21-16(15)22-12-23-17/h1-5,8,12H,6-7,9-11,20H2,(H,21,22,23)
|
| 化学名 |
4-(4-chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidin-4-amine
|
| 别名 |
CCT128930; CCT-128930; CCT128930; 885499-61-6; 4-(4-chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidin-4-amine; CCT-128,930; 4-[(4-chlorophenyl)methyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidin-4-amine; CHEMBL263664; 4-[(4-chlorophenyl)methyl]-1-{7H-pyrrolo[2,3-d]pyrimidin-4-yl}piperidin-4-amine; CCT 128,930; CCT 128930
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (6.08 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (6.08 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (6.08 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 1% DMSO+30% polyethylene glycol+1% Tween 80: 11mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9253 mL | 14.6267 mL | 29.2535 mL | |
| 5 mM | 0.5851 mL | 2.9253 mL | 5.8507 mL | |
| 10 mM | 0.2925 mL | 1.4627 mL | 2.9253 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Effect of CCT128930 exposure on expression of AKT biomarkers and cell cycle proteins in a panel of human tumor cell lines. Mol Cancer Ther. 2011, 10(2), 360-371. |
Pharmacokinetic behavior and pharmacodynamic effects of CCT128930 in vivo. |
Antitumor activity of CCT128930 in human tumor xenografts. |