| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
α2-adrenergic receptor
|
|---|---|
| 体外研究 (In Vitro) |
可乐定抑制神经元的放电能力[5]。中缝背核(DR)是下脑干中线的一个核系统,被认为是中枢神经系统疼痛调节中最重要的核之一。中枢去甲肾上腺素能系统在心血管调节和疼痛传递的控制中起着重要作用。可乐定是一种α2-肾上腺素能激动剂,广泛用于麻醉研究。在这项研究中,我们评估了可乐定对DR核活性的影响及其在疼痛调节中的可能作用。使用细胞外记录技术测试了大鼠脑干切片制备中DR核内的74个神经元。应用50μmol/L的去甲肾上腺素(NA)诱导68个受试神经元(92%)的放电活动。NA在DR神经元上产生了有规律的持久放电活动。先前被NA激发的56个神经元(88%)被20 mumol/L的可乐定抑制。可乐定可抑制神经元的放电活动。结果表明,DR神经元的放电受到去甲肾上腺素能的影响,并被可乐定抑制,可乐定进而通过改变中枢5-羟色胺能系统来改变伤害性感受[5]。 |
| 体内研究 (In Vivo) |
可乐定 (50 μg/kg,腹腔注射) 诱导大鼠体温显着降低,持续 3 小时,给药后 1 小时达到最大值。在可乐定前 15 分钟用中性剂量的酚妥拉明对大鼠进行脑室内预处理,可显着拮抗可乐定引起的体温过低[1]。 Clonidine (0.003-0.05 mg/kg, ip) 有效抑制 PCP 诱导的前额皮质多巴胺流出。使用 α-2A 受体拮抗剂 (BRL-44408) 进行预处理可防止可乐定抑制 PCP 诱导的前额皮质多巴胺溢出 [3]。在 DMSO 预处理的 SO 大鼠中,可乐定(0.6 μg ic)对血压没有影响。然而,在 SO 大鼠中进行中枢腺苷 A1R 阻断 (DPCPX) 后,可乐定显着(P < 0.05,单向方差分析)降低血压。相比之下,在 DMSO 预处理的 ABD 大鼠中,可乐定(0.6 μg ic)可导致血压显着降低;重要的是,中枢 A1R 阻断(DPCPX 预处理)不会影响(P > 0.05,单向方差分析)可乐定引起的 ABD 大鼠血压降低。在 DPCPX 预处理的 SO 大鼠中,随着低血压反应的出现,与 DMSO 预处理的 SO 大鼠中的基础或可乐定治疗相比,可乐定导致 RVLM pERK1/2 水平显着增加 (P < 0.05)。在媒介物 (DMSO) 预处理的 ABD 大鼠中,可乐定显着 (P < 0.05) 增强 RVLM pERK1/2,并且这种反应不受 DPCPX 预处理的影响[4]。
|
| 酶活实验 |
N-甲基-D-天冬氨酸/谷氨酸受体拮抗剂在人类和动物中诱导拟精神作用,许多研究都集中在介导这些行为变化的神经化学和网络水平效应上。例如,NMDA依赖性谷氨酸能传递的减少会触发单胺递质的释放增加,其中一些变化与苯环利定(PCP)的认知、行为和神经解剖学效应有关。α-2肾上腺素受体激动剂(如可乐定)能有效预防NMDA拮抗剂的许多行为、神经化学和解剖学效应。有证据表明,可乐定诱导NMDA拮抗剂作用逆转的一个关键机制是减弱增强的多巴胺释放。我们通过研究α-2激动剂对自由运动大鼠前额叶皮层PCP诱发的多巴胺外流的影响来追求这些发现。可乐定(0.003-0.1mg/kg,i.p.)剂量依赖性地减弱了五氯苯酚(2.5mg/kg,i.p..)增加皮质多巴胺输出的能力。可乐定的作用被α-2A亚型选择性拮抗剂BRL-44408(1mg/kg,i.p.)阻止。Guanfacine是一种α-2激动剂,与2B或2C亚型相比,它对2A的亲和力更高,也阻断了PCP增加前额叶皮层多巴胺外流的能力。这些数据表明,α-2A激动剂能有效抵消PCP诱导的高多巴胺能状态,并可能在这种假定的精神分裂症动物模型中的神经行为效应中发挥作用[4]。
|
| 细胞实验 |
本研究旨在确定可乐定是否可以诱导降钙素基因相关肽(CGRP)的产生及其潜在机制。用可乐定处理人脐静脉内皮细胞,并研究可乐定对CGRP产生的剂量-效应或时间-效应关系。选择育亨宾(一种α2肾上腺素受体阻断剂)和L-NAME(一种一氧化氮合酶(NOS)拮抗剂)来探索α2肾上腺素能受体和一氧化氮途径在可乐定对内皮细胞来源的CGRP产生的影响中的作用。采用实时荧光定量PCR或放射免疫法检测CGRP mRNA或蛋白水平。采用硝基还原法测定一氧化氮含量。研究表明,可乐定能够以剂量依赖的方式诱导内皮细胞中CGRP mRNA(α和β亚型)的表达。在育亨宾存在的情况下,可乐定对内皮细胞来源的CGRP合成和分泌的影响减弱。L-NAME治疗还可以抑制可乐定诱导的CGRP合成和分泌,同时降低培养基中的NO含量。这些结果表明,可乐定可以通过激活α2肾上腺素受体来刺激内皮细胞中CGRP的合成和分泌,这与NO途径有关[3]。
|
| 动物实验 |
On the day of the experiment, two hours before the baseline sample collection starts, the flow rate is increased to 2 μL/min. Following the collection of four baseline samples, animals are pretreated with an intraperitoneal (i.p.) injection of either 0.9% saline (the vehicle), clonidine (0.0033, 0.01, or 0.05 mg/kg), or guanfacine (0.05 or 0.5 mg/kg). Twenty minutes later, the animals receive an injection of PCP (2.5 mg/kg, i.p.). Dialysates are collected every twenty minutes. BRL (1.0 mg/kg) is given 20 minutes before clonidine in a different study. Furthermore, in certain control studies, the animals are given a single injection of saline, clonidine (0.01 or 0.05 mg/kg), guanfacine (0.5 mg/kg), or BRL (1.0 mg/kg).
Central adenosine A(1) and A(2A) receptors mediate pressor and depressor responses, respectively. The adenosine subtype A(2A) receptor (A(2A)R)-evoked enhancement of phosphorylated extracellular signal-regulated kinase (pERK) 1/2 production in the rostral ventrolateral medulla (RVLM), a major neuroanatomical target for clonidine, contributes to clonidine-evoked hypotension, which is evident in conscious aortic barodenervated (ABD) but not in conscious sham-operated (SO) normotensive rats. We conducted pharmacological and cellular studies to test the hypothesis that the adenosine A(2A)R-mediated (pERK1/2-dependent) hypotensive action of clonidine is not expressed in SO rats because it is counterbalanced by fully functional central adenosine subtype A(1) receptor (A(1)R) signaling. We first demonstrated an inverse relationship between A(1)R expression in RVLM and clonidine-evoked hypotension in ABD and SO rats. The functional (pharmacological) relevance of the reduced expression of RVLM A(1)R in ABD rats was verified by the smaller dose-dependent pressor responses elicited by the selective A(1)R agonist N(6)-cyclopentyladenosine in ABD versus SO rats. It is important that after selective blockade of central A(1)R with 8-cyclopentyl-1,3-dipropylxanthine in conscious SO rats, clonidine lowered blood pressure and significantly increased neuronal pERK1/2 in the RVLM. In contrast, central A(1)R blockade had no influence on the hypotensive response or the increase in RVLM pERK1/2 elicited by clonidine in ABD rats. These findings support the hypothesis that central adenosine A(1)R signaling opposes the adenosine A(2A)R-mediated (pERK1/2-dependent) hypotensive response and yield insight into a cellular mechanism that explains the absence of clonidine-evoked hypotension in conscious normotensive rats.[2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Clonidine reaches maximum concentration in 60-90 minutes after oral administration. Race and fasting status do not influence pharmacokinetics of clonidine. A 100µg oral clonidine tablet reaches a Cmax of 400.72pg/mL with an AUC of 5606.78h\*pg/mL and a bioavailability of 55-87%. Approximately 50% of a clonidine dose is excreted in the urine as the unchanged drug and 20% is eliminated in the feces. The volume of distribution of clonidine has been reported as 1.7-2.5L/kg, 2.9L/kg, or 2.1±0.4L/kg depending on the source. The clearance of clonidine is 1.9-4.3mL/min/kg. Animal studies indicate that clonidine is widely distributed into body tissues; tissue concentrations of the drug are higher than plasma concentrations. The mean volume of distribution of clonidine is reported to be 2.1 L/kg. After oral administration, highest concentrations of the drug are found in the kidneys, liver, spleen, and GI tract. High concentrations of the drug also appear in the lacrimal and parotid glands. Clonidine is concentrated in the choroid of the eye and is also distributed into the heart, lungs, testes, adrenal glands, fat, and muscle. The lowest concentration occurs in the brain. Clonidine is distributed into CSF. Following epidural infusion, clonidine is rapidly and extensively distributed into CSF and readily partitions into the plasma via epidural veins. In vitro, clonidine is approximately 20-40% bound to plasma proteins, mainly albumin. Clonidine crosses the placenta1 and is distributed into milk. In one lactating woman who received approximately 0.04 mg of oral clonidine hydrochloride twice daily and 25 mg of oral dihydralazine 3 times daily, clonidine concentrations were 0.33 ng/mL in a plasma sample obtained 1 hour after a dose and 0.6 ng/mL in a milk sample obtained 2.5 hours after a dose; the drug was not detected in the plasma of the infant 1 hour after nursing. ...IN HEALTHY VOLUNTEERS... AFTER IV INFUSION OF 300 UG CLONIDINE IN 10 MINUTES, PLASMA LEVELS OF DRUG DECLINED BI-EXPONENTIALLY WITH RAPID AND SLOW HALF-LIFE VALUES OF 11 MINUTES AND 8.5 HOURS, RESPECTIVELY. THE PHARMACOKINETICS OF CLONIDINE WERE INVESTIGATED IN HEALTHY VOLUNTEERS OVER A TIME MORE THAN 3 TIMES LONGER THAN PREVIOUSLY REPORTED. APPROXIMATELY 62% OF A GIVEN DOSE WAS EXCRETED UNCHANGED IN URINE, INDEPENDENT OF THE QUANTITY ADMINISTERED, THE DRUG FORMULATION, OR THE MODE OF ADMINISTRATION. SINCE THE PHARMACOKINETICS OF THE DRUG WERE AFFECTED BY ENTEROHEPATIC CIRCULATION, IT CANNOT BE DESCRIBED BY A CONVENTIONAL, OPEN 1 OR 2 COMPARTMENT MODEL. THE TIME COURSES OF THE PLASMA CLONIDINE CONCENTRATION AND ITS DRUG EFFECTS RAN ASYNCHRONOUSLY. CLONIDINE KINETICS WERE STUDIED IN 21 PATIENTS WITH ESSENTIAL HYPERTENSION WHO RECEIVED 2 BOLUS IV INJECTIONS (0.78-3.36 MCG/KG) AND ONE SINGLE ORAL DOSE (1.7-2.3 MCG/KG) ON SEPARATE OCCASIONS; KINETICS WERE STUDIED IN SOME PATIENTS AFTER MULTIPLE THERAPEUTIC ORAL DOSES (1.1 OR 1.9 MCG/KG TWICE DAILY) DURING A DOSAGE INTERVAL AFTER 6-12 MONTHS MONOTHERAPY WITH CLONIDINE. WITH INCREASING IV DOSES, THE RATE CONSTANTS DECREASED AND THE PLASMA CLEARANCE WAS REDUCED BY 74% (9.94-2.61 ML/MIN/KG) INDICATING DOSE-DEPENDENT KINETICS. THE VOLUME OF DISTRIBUTION DID NOT CHANGE WITH DOSE IN CONTRAST TO THE VOLUME OF THE PLASMA COMPARTMENT WHICH WAS INCREASED AT THE HIGHEST DOSES. THE SINGLE ORAL DOSE KINETICS AGREED WITH THE IV KINETICS AT COMPARABLE DOSE. THE BIOAVAILABILITY WAS 90%. DURING MULTIPLE ORAL DOSING THE ELIMINATION RATE CONSTANTS DECREASED COMPARED TO THE SINGLE DOSE. THE PLASMA CLEARANCE INCREASED (7.18 ML/MIN/KG) COMPARED TO THE CORRESPONDING SINGLE DOSE (4.17 ML/MIN/KG). THE LATTER CHANGE WAS PROBABLY CAUSED BY THE DECREASE IN BIOAVAILABILITY TO ABOUT 65%. IT WAS DETERMINED THAT THE PHARMACODYNAMIC PROPERTIES OF THE DRUG COULD EXPLAIN THE CHANGES IN PHARMACOKINETICS WITH INCREASED DOSE AND DURING MULTIPLE DOSES. For more Absorption, Distribution and Excretion (Complete) data for CLONIDINE (8 total), please visit the HSDB record page. Metabolism / Metabolites The metabolism of clonidine is poorly understood. The main reaction in clonidine metabolism is the 4-hydroxylation of clonidine by CYP2D6, CYP1A2, CYP3A4, CYP1A1, and CYP3A5. Clonidine is <50% metabolized in the liver to inactive metabolites. Clonidine hydrochloride is metabolized in the liver. In humans, 4 metabolites have been detected but only one, the inactive p-hydroxyclonidine, has been identified. ...Seventeen cDNA-expressed P450 enzymes, in addition to pooled human liver microsomes, were evaluated for clonidine 4-hydroxylation activity in vitro. Five P450 enzymes-CYP2D6, 1A2, 3A4, 1A1, and 3A5-catalyzed measurable formation of 4-hydroxyclonidine. Selective inhibition studies in human liver microsomes confirmed that these isoforms are jointly responsible for 4-hydroxylation of clonidine in vitro, and CYP2D6 accounted for approximately two-thirds of the activity. The major role of CYP2D6 in clonidine metabolism might explain the increase in its nonrenal clearance during pregnancy. CLONIDINE SHOWS SPECIES DIFFERENCES IN THE EXTENT OF BIOTRANSFORMATION. THE FATE OF (14)C-CLONIDINE IN THE DOG HAS BEEN REPORTED AND SIX COMPONENTS WERE ISOLATED AND IDENTIFIED. UNCHANGED CLONIDINE AND ITS P-HYDROXYLATED DERIVATIVE WERE DETECTED. DICHLOROPHENYLGUANIDINE, WHICH HAS PREVIOUSLY BEEN REPORTED AS A METABOLITE IN DOGS, WAS ALSO IDENTIFIED. THREE METABOLITES NOT PREVIOUSLY DESCRIBED WERE ALSO ISOLATED FROM DOG URINE. THE MAJOR METABOLIC ROUTES FOR CLONIDINE ARE PHENYL RING HYDROXYLATION AND SPLITTING OF THE IMIDAZOLIDINE RING. COMPARATIVE STUDIES SHOWED THAT THE METABOLISM OF CLONIDINE IS RATHER SIMILAR IN RAT, DOG, AND MAN, BUT MAN EXCRETED MOST UNCHANGED DRUG AND DOG SHOWED THE MOST EXTENSIVE METABOLISM. Hepatic. Metabolized via minor pathways. The major metabolite, p-hydroxyclonidine, is present in concentrations less than 10% of those of unchanged clonidine in urine. Four metabolites have been detected, but only p-hydroxyclonidine has been identified. Half Life: 6-20 hours; 40-60% is excreted in urine unchanged, 20% is excreted in feces. Less than 10% is excreted by p-hydroxyclonidine. Biological Half-Life The elimination half life after epidural administration is 30 minutes but otherwise can range from 6-23h. The elimination half-life of the drug ranges from 6 to 24 hours, with a mean of about 12 hours. THE PHARMACOKINETICS OF CLONIDINE WERE INVESTIGATED IN HEALTHY VOLUNTEERS OVER A TIME MORE THAN 3 TIMES LONGER THAN PREVIOUSLY REPORTED. THE COMPLETE BIOAVAILABILITY OF CLONIDINE AND ITS ELIMINATION T/2 (20 TO 25.5 HOURS) REMAINED CONSTANT AFTER SINGLE AND MULTIPLE DOSES. The plasma half-life of clonidine is 6-20 hours in patients with normal renal function. The half-life in patients with impaired renal function has been reported to range from 18-41 hours. The elimination half-life of the drug may be dose dependent, increasing with increasing dose. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Clonidine acts as an agonist at presynaptic alpha(2)-receptors in the nucleus tractus solitarius of the medulla oblongata. Stimulation of these receptors results in the supression of efferent sympathetic pathways and the subsequent decrease in blood pressure and vascular tone in the heart, kidneys, and peripheral vasculature. Clonidine is also a partial agonist at presynaptic alpha(2)-adrenergic receptors of peripheral nerves in vascular smooth muscle. Toxicity Data LD50: 150 mg/kg (oral, rat) LD50: 30 mg/kg (oral, dog) Interactions Potential additive effects (eg, hypotension, bradycardia). If carvedilol is used concomitantly with clonidine, caution should be exercised, particularly when discontinuing therapy; carvedilol generally should be discontinued first, and clonidine continued for several days thereafter with gradual downward dosage titration. Epidural clonidine may prolong the duration of the pharmacologic effects, including both sensory and motor blockade, of epidural local anesthetics. Because beta-adrenergic blocking agents may exacerbate rebound hypertension that may occur following discontinuance of clonidine therapy, beta-adrenergic blocking agents should be discontinued several days before gradual withdrawal of clonidine when clonidine therapy is to be discontinued in patients receiving a beta-adrenergic blocking agent and clonidine concurrently. If clonidine therapy is to be replaced by a beta-adrenergic blocking agent, administration of the beta-adrenergic blocking agent should be delayed for several days after clonidine therapy has been discontinued Because clonidine may produce bradycardia and atrioventricular (AV) block, the possibility of additive effects should be considered if it is given concomitantly with other drugs that affect sinus node function or AV nodal conduction (e.g., guanethidine), beta-adrenergic blocking agents (e.g., propranolol), calcium-channel blocking agents, or cardiac glycosides. For more Interactions (Complete) data for CLONIDINE (15 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat oral 126 mg/kg /Clonidine hydrochloride/ LD50 Rat ip 100 mg/kg /Clonidine hydrochloride/ LD50 Rat iv 29 mg/kg /Clonidine hydrochloride/ LD50 Rat sc 77 mg/kg /Clonidine hydrochloride/ For more Non-Human Toxicity Values (Complete) data for CLONIDINE (9 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Adrenergic alpha-Agonists; Antihypertensive Agents; Sympatholytics; Analgesics Clonidine hydrochloride and transdermal clonidine are used alone or in combination with other classes of antihypertensive agents in the management of hypertension. /Included in US product labeling/ Clonidine hydrochloride administered by epidural infusion is used as adjunctive therapy in combination with opiates in the management of severe cancer pain that is not relieved by opiate analgesics alone. /Clonidine hydrochloride; Included in US product labeling/ Oral loading-dose regimens of clonidine hydrochloride have been effective in rapidly reducing blood pressure in patients with severe hypertension in whom reduction of blood pressure was considered urgent, but not requiring emergency treatment. Hypertensive urgencies are those situations in which it is desirable to reduce blood pressure within a few hours. Such situations include the upper levels of severe hypertension, hypertension with optic disk edema, progressive target organ complications, and severe perioperative hypertension. /Clonidine hydrochloride; NOT included in US product labeling/ For more Therapeutic Uses (Complete) data for CLONIDINE (15 total), please visit the HSDB record page. Drug Warnings Abrupt withdrawal of clonidine therapy may result in a rapid increase of systolic and diastolic blood pressures with associated symptoms such as nervousness, agitation, confusion, restlessness, anxiety, insomnia, headache, sweating, palpitation, increased heart rate, tremor, hiccups, stomach pains, nausea, muscle pains, and increased salivation. The exact mechanism(s) of the withdrawal syndrome following discontinuance of alpha-adrenergic agonists has not been determined but may involve increased concentrations of circulating catecholamines, increased sensitivity of adrenergic receptors, enhanced renin-angiotensin system activity, decreased vagal function, failure of autoregulation of cerebral blood flow, and/or failure of central alpha2-adrenergic receptor mechanisms that regulate sympathetic outflow from the CNS and modulate baroreflex function. Because of the risk of rebound hypertension, patients receiving clonidine preparations should be warned of the danger of missing doses or stopping the drug without consulting their physician. When discontinuing clonidine therapy, a rapid rise in blood pressure may be minimized or prevented by tapered withdrawal of the drug over 2-4 days. Tapered withdrawal of transdermal clonidine or initiation of a tapered regimen of oral clonidine also is recommended by some clinicians when the transdermal dosage form is discontinued, particularly in geriatric patients. If clonidine therapy is to be discontinued in patients receiving clonidine and a beta-adrenergic blocking agent concomitantly, the beta-adrenergic blocker should be discontinued several days before clonidine therapy is discontinued. It is recommended that clonidine therapy not be interrupted for surgery; transdermal therapy can be continued throughout the perioperative period and oral therapy should be continued to within 4 hours before surgery. Blood pressure should be carefully monitored during surgery and additional measures to control blood pressure should be available if necessary. If clonidine therapy must be interrupted for surgery, parenteral hypotensive therapy should be administered as necessary, and clonidine therapy should be resumed as soon as possible. If transdermal therapy is initiated during the perioperative period, it must be kept in mind that therapeutic plasma clonidine concentrations are not achieved until 2-3 days after initial application of the transdermal system. Implantable epidural catheters are associated with a risk of infection, including meningitis and/or epidural abscess. The incidence of catheter-related infections is about 5-20%, and depends on several factors, including the clinical status of the patient, type of catheter used, catheter placement technique, quality of catheter care, and duration of catheter placement. The possibility of catheter-related infection should be considered in patients receiving epidural clonidine who develop a fever. Fever, malaise, pallor, muscle or joint pain, and leg cramps have been reported in up to 0.5% of patients during postmarketing experience with transdermal clonidine. For more Drug Warnings (Complete) data for CLONIDINE (22 total), please visit the HSDB record page. Pharmacodynamics Clonidine functions through agonism of alpha-2 adrenoceptors which have effects such as lowering blood pressure, sedation, and hyperpolarization of nerves. It has a long duration of action as it is given twice daily and the therapeutic window is between 0.1mg and 2.4mg daily. |
| 分子式 |
C9H9CL2N3
|
|---|---|
| 分子量 |
230.094
|
| 精确质量 |
229.017
|
| 元素分析 |
C, 46.98; H, 3.94; Cl, 30.82; N, 18.26
|
| CAS号 |
4205-90-7
|
| 相关CAS号 |
Clonidine hydrochloride;4205-91-8;Clonidine-d4 hydrochloride;67151-02-4
|
| PubChem CID |
2803
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
319.3±52.0 °C at 760 mmHg
|
| 熔点 |
141-142℃
|
| 闪点 |
146.9±30.7 °C
|
| 蒸汽压 |
0.0±0.7 mmHg at 25°C
|
| 折射率 |
1.671
|
| LogP |
1.41
|
| tPSA |
36.42
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
14
|
| 分子复杂度/Complexity |
222
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
GJSURZIOUXUGAL-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C9H9Cl2N3/c10-6-2-1-3-7(11)8(6)14-9-12-4-5-13-9/h1-3H,4-5H2,(H2,12,13,14)
|
| 化学名 |
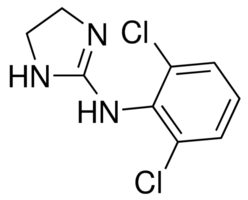
N-(2,6-dichlorophenyl)-4,5-dihydro-1H-imidazol-2-amine
|
| 别名 |
clonidine; 4205-90-7; Clonidin; Chlornidinum; N-(2,6-Dichlorophenyl)-4,5-dihydro-1H-imidazol-2-amine; Catapres-TTS; Adesipress; Catapres;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~434.61 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (10.87 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (10.87 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (10.87 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.3461 mL | 21.7306 mL | 43.4613 mL | |
| 5 mM | 0.8692 mL | 4.3461 mL | 8.6923 mL | |
| 10 mM | 0.4346 mL | 2.1731 mL | 4.3461 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Treatment of Neonatal Abstinence Syndrome With Clonidine Versus Morphine as Primary Therapy
CTID: NCT03092011
Phase: Phase 4 Status: Active, not recruiting
Date: 2024-10-15