| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
PRMT5
|
|---|---|
| 体外研究 (In Vitro) |
尽管 CMP-5(0-100 μM;24-72 小时)会发挥作用,但即使经过较长时间,其对正常静息 B 的毒性也是有限的 [1]。与用 DMSO 处理的组相比,CMP-5(40 μM;24 小时)降低了 60A 细胞中 p-BTK 和 pY (416) SRC 的表达 [1]。当暴露于 CMP-5(0–40 μM;24 小时)时,PRMT5 优先在 Th1 细胞中转录,而不是 Th2 细胞;在人 Th1 细胞和 Th2 细胞中,IC50 值分别为 26.9 μM 和 31.6 μM。 CMP-5(25 μM;24 小时)使小鼠 Th1 细胞增殖降低 91%。应用不同剂量的IL-2,IL-2增强增殖的最大程度在5ng/mL时观察到[1]。
|
| 体内研究 (In Vivo) |
抑制PRMT5抑制ova诱导的DTH炎症反应[2]
PRMT5抑制剂抑制炎症记忆T细胞反应的有效性表明它们可能对炎症或自身免疫性疾病有益。为了验证这一点,我们使用了OVA诱导的DTH小鼠模型和HLCL65,这是一种更有效和生物可利用的CMP5的衍生物(图1H, 2H,补充图2)。首先,我们分析了CFA免疫OVA后未治疗小鼠脾脏中PRMT5的表达。我们观察到,在免疫后10 d, PRMT5在脾脏中的表达显著上调(图6A),这表明PRMT5的表达与体内DTH免疫应答有关。在DTH模型中(如图6B所示),用CFA免疫OVA诱导OVA特异性T细胞反应,在随后暴露于无佐剂的OVA和记忆性CD4+ T细胞扩增后,引起小鼠足垫炎症。在再挑战期间,HLCL65治疗减少了40%的足底肿胀(炎症的衡量标准)(p < 0.05,图6C)。此外,与载体相比,HLCL65处理降低了36%的ova特异性T细胞增殖(图6D)和70%的IFN-γ产生(图6E)。这些数据表明,我们的新型PRMT5抑制剂HLCL65在体内抑制T细胞介导的反应和炎症。 |
| 细胞实验 |
蛋白质印迹分析 [1]
细胞类型: 60A 细胞 测试浓度: 40 μM 孵育时间: 24 hrs (hrs (小时)) 实验结果: p-BTK 和 pY(416)SRC 蛋白水平的抑制。 细胞活力测定 [1] 细胞类型:人类 Th1 细胞和 Th2 细胞 测试浓度: 25 μM 孵育持续时间:24小时 实验结果:抑制小鼠Th1细胞增殖,但添加IL-2剂量依赖性地增加细胞增殖。 |
| 动物实验 |
OVA-induced DTH [2]
CFA and OVA emulsion was prepared at a 1:1 v/v ratio for a final concentration of 1500 μg of OVA/1 ml of PBS. BALB/c mice were injected with 100 μl of emulsion in the dorsal proximal scruff and the base of the tail (150 μg of OVA per mouse). Control groups included nonimmunized mice and immunized mice that were not subsequently challenged with OVA. One week after immunization, aggregated OVA was prepared by suspending in PBS at a concentration of 10 mg/ml in a 15-ml tube. Solution was heated in an 80°C water bath for 60 min. Mice were challenged with 300 μg of aggregated OVA by injecting 30 μl of solution into the left footpad of immunized mice. After an additional week, mice were rechallenged in the same manner (nonimmunized mice were also challenged at this step). Twenty-four hours after the second challenge, mice were euthanized by CO2 asphyxiation and cervical dislocation. Each footpad was measured using calipers for swelling (pre-euthanasia) and weighed for changes in mass. Additionally, spleens were removed and processed for in vitro studies. Experimental autoimmune encephalomyelitis [2] For induced EAE, commercial Hooke Reagent or myelin oligodendrocyte glycoprotein and CFA emulsion were used. CFA/MOG emulsion was prepared in a 1:1 v/v ratio for a final concentration of 1000 μg MOG/1 ml of PBS. C57/B6 mice received 100 μl of emulsion s.c. in the dorsal proximal scruff and the base of the tail. About 2 h after immunization, mice were injected i.p. with 100 μl of 2 ng/μl pertussis toxin. Twenty-four hours later, mice were injected again with 100 μl of 2 ng/μl pertussis toxin. Mice were monitored for disease every day and treated with 25 mg/kg HLCL65 or DMSO vehicle control. At the indicated time points, mice were euthanized by injection with 20 mg/ml ketamine and 4 mg/ml xylazine (120 μl/20 g mouse) and perfused with PBS. Spleens, brains, and spinal cords were collected from representative mice and processed for in vitro studies. To isolate brain and spinal cord mononuclear cells, brains and spinal cords were processed through a 70-μm strainer and separated by a 70–30% isotonic Percoll gradient. [2] For spontaneous EAE, three MBPAc1–11 TCR-Tg mice that developed EAE spontaneously (scores = 1.5–2) were euthanized by CO2 asphyxiation and cervical dislocation. Splenocytes were isolated and activated with 2 μg/ml MBPAc1–11 for 48 h in the presence of PRMT5 inhibitors or vehicle control. T-bet, IL-17, and RORγt expression was analyzed by intracellular flow cytometry. |
| 参考文献 |
|
| 其他信息 |
Epigenetic events that are essential drivers of lymphocyte transformation remain incompletely characterized. We used models of Epstein-Barr virus (EBV)-induced B-cell transformation to document the relevance of protein arginine methyltransferase 5 (PRMT5) to regulation of epigenetic-repressive marks during lymphomagenesis. EBV(+) lymphomas and transformed cell lines exhibited abundant expression of PRMT5, a type II PRMT enzyme that promotes transcriptional silencing of target genes by methylating arginine residues on histone tails. PRMT5 expression was limited to EBV-transformed cells, not resting or activated B lymphocytes, validating it as an ideal therapeutic target. We developed a first-in-class, small-molecule PRMT5 inhibitor that blocked EBV-driven B-lymphocyte transformation and survival while leaving normal B cells unaffected. Inhibition of PRMT5 led to lost recruitment of a PRMT5/p65/HDAC3-repressive complex on the miR96 promoter, restored miR96 expression, and PRMT5 downregulation. RNA-sequencing and chromatin immunoprecipitation experiments identified several tumor suppressor genes, including the protein tyrosine phosphatase gene PTPROt, which became silenced during EBV-driven B-cell transformation. Enhanced PTPROt expression following PRMT5 inhibition led to dephosphorylation of kinases that regulate B-cell receptor signaling. We conclude that PRMT5 is critical to EBV-driven B-cell transformation and maintenance of the malignant phenotype, and that PRMT5 inhibition shows promise as a novel therapeutic approach for B-cell lymphomas.[1]
In the autoimmune disease multiple sclerosis and its animal model, experimental autoimmune encephalomyelitis (EAE), expansion of pathogenic, myelin-specific Th1 cell populations drives active disease; selectively targeting this process may be the basis for a new therapeutic approach. Previous studies have hinted at a role for protein arginine methylation in immune responses, including T cell-mediated autoimmunity and EAE. However, a conclusive role for the protein arginine methyltransferase (PRMT) enzymes that catalyze these reactions has been lacking. PRMT5 is the main PRMT responsible for symmetric dimethylation of arginine residues of histones and other proteins. PRMT5 drives embryonic development and cancer, but its role in T cells, if any, has not been investigated. In this article, we show that PRMT5 is an important modulator of CD4+ T cell expansion. PRMT5 was transiently upregulated during maximal proliferation of mouse and human memory Th cells. PRMT5 expression was regulated upstream by the NF-κB pathway, and it promoted IL-2 production and proliferation. Blocking PRMT5 with novel, highly selective small molecule PRMT5 inhibitors severely blunted memory Th expansion, with preferential suppression of Th1 cells over Th2 cells. In vivo, PRMT5 blockade efficiently suppressed recall T cell responses and reduced inflammation in delayed-type hypersensitivity and clinical disease in EAE mouse models. These data implicate PRMT5 in the regulation of adaptive memory Th cell responses and suggest that PRMT5 inhibitors may be a novel therapeutic approach for T cell-mediated inflammatory disease.[2] |
| 分子式 |
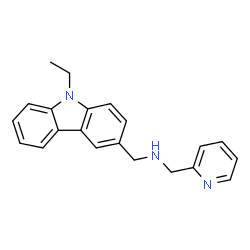
C₂₁H₂₁N₃
|
|---|---|
| 分子量 |
315.41
|
| 精确质量 |
315.173
|
| CAS号 |
880813-42-3
|
| 相关CAS号 |
CMP-5 hydrochloride;1030021-40-9
|
| PubChem CID |
4722595
|
| 外观&性状 |
Light yellow to yellow ointment
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
505.1±48.0 °C at 760 mmHg
|
| 闪点 |
259.3±29.6 °C
|
| 蒸汽压 |
0.0±1.3 mmHg at 25°C
|
| 折射率 |
1.637
|
| LogP |
4.23
|
| tPSA |
29.8
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
399
|
| 定义原子立体中心数目 |
0
|
| SMILES |
N1C(CNCC2C=C3C4C(N(C3=CC=2)CC)=CC=CC=4)=CC=CC=1
|
| InChi Key |
YPJMOVVQKBFRNH-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C21H21N3/c1-2-24-20-9-4-3-8-18(20)19-13-16(10-11-21(19)24)14-22-15-17-7-5-6-12-23-17/h3-13,22H,2,14-15H2,1H3
|
| 化学名 |
1-(9-ethylcarbazol-3-yl)-N-(pyridin-2-ylmethyl)methanamine
|
| 别名 |
CMP5; cmp-5; 880813-42-3; 1-(9-ethyl-9H-carbazol-3-yl)-N-(pyridin-2-ylmethyl)methanamine; 1-(9-ethylcarbazol-3-yl)-N-(pyridin-2-ylmethyl)methanamine; CHEMBL4245087; SCHEMBL21308321; (9-Ethyl-9H-carbazol-3-ylmethyl)-pyridin-2-ylmethyl-amine; CMP 5
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~125 mg/mL (~396.31 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 6.25 mg/mL (19.82 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 62.5 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 6.25 mg/mL (19.82 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 62.5 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1705 mL | 15.8524 mL | 31.7048 mL | |
| 5 mM | 0.6341 mL | 3.1705 mL | 6.3410 mL | |
| 10 mM | 0.3170 mL | 1.5852 mL | 3.1705 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。