| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
Cyclizine (100 μM) 显着降低 RAW 264.7 细胞上清液中 iNOS 蛋白和 LPS 刺激的亚硝酸盐积累的水平 [1]。环己哌啶的 IC50 为 5.42 µM,可防止抗 IgE 诱导的人肺碎片释放组胺[2]。
|
|---|---|
| 体内研究 (In Vivo) |
cyclolizine (1, 10 mg/kg) 以剂量依赖性方式增强小鼠的运动活动 [3]。
|
| 动物实验 |
Animal/Disease Models: Naive male CDI mice (Charles River), body weight 25-30 g[3]
Doses: 1, 10 mg/kg Route of Administration: subcutaneous injection; measure locomotor activity (crossover) every 15 minutes for 2 hrs (hrs (hours)) . Experimental Results: Enhanced locomotor activity in a dose-dependent manner. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
BENZHYDROLPIPERAZINES & THEIR N-DEALKYLATION PRODUCTS ARE DISTRIBUTED IN ALL TISSUES OF RAT & ARE TRANSFERRED TO FETUS. /BENZYHYDROLPIPERAZINES/ Metabolism / Metabolites Cyclizine is metabolised to its N-demethylated derivative, norcyclizine, which has little antihistaminic (H1) activity compared to Cyclizine. OXIDATIVE N-DEALKYLATION IS MAIN METABOLIC PATHWAY OF BENZHYDROLPIPERAZINES; CYCLIZINE.../IS/ TRANSFORMED INTO NORCYCLIZINE... WITH A PURIFIED MIXED FUNCTION OXIDASE FROM LIVER MICROSOMES IN PRESENCE OF REDUCED PYRIDINE NUCLEOTIDE & O2.../CYCLIZINE IS/ OXIDIZED TO N-OXIDE. Biological Half-Life 20 hours |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Occasional doses of cyclizine are probably acceptable during breastfeeding. Large doses or more prolonged use may cause effects in the infant or decrease the milk supply, particularly in combination with a sympathomimetic such as pseudoephedrine or before lactation is well established. ◉ Effects in Breastfed Infants Relevant published information on cyclizine was not found as of the revision date. In one telephone follow-up study, mothers reported irritability and colicky symptoms 10% of infants exposed to various antihistamines and drowsiness was reported in 1.6% of infants. None of the reactions required medical attention and none of the infants were exposed to cyclizine. ◉ Effects on Lactation and Breastmilk Antihistamines in relatively high doses given by injection can decrease basal serum prolactin in nonlactating women and in early postpartum women. However, suckling-induced prolactin secretion is not affected by antihistamine pretreatment of postpartum mothers. Whether lower oral doses of antihistamines have the same effect on serum prolactin or whether the effects on prolactin have any consequences on breastfeeding success have not been studied. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Interactions COMBINATIONS OF CAFFEINE 100 MG WITH CYCLIZINE-HCL 50 & 100 MG DID NOT PRODUCE ANY SUBJECTIVE CHANGES OR CHANGES IN PERFORMANCE TESTS, DIFFERING FROM CONTROL. |
| 参考文献 |
|
| 其他信息 |
Cyclizine is an N-alkylpiperazine in which one nitrogen of the piperazine ring is substituted by a methyl group, while the other is substituted by a diphenylmethyl group. It has a role as an antiemetic, a cholinergic antagonist, a central nervous system depressant, a local anaesthetic and a H1-receptor antagonist.
A histamine H1 antagonist given by mouth or parenterally for the control of postoperative and drug-induced vomiting and in motion sickness. (From Martindale, The Extra Pharmacopoeia, 30th ed, p935) A histamine H1 antagonist given by mouth or parenterally for the control of postoperative and drug-induced vomiting and in motion sickness. (From Martindale, The Extra Pharmacopoeia, 30th ed, p935) Drug Indication For prevention and treatment of nausea, vomiting, and dizziness associated with motion sickness, and vertigo (dizziness caused by other medical problems). Mechanism of Action Vomiting (emesis) is essentially a protective mechanism for removing irritant or otherwise harmful substances from the upper GI tract. Emesis or vomiting is controlled by the vomiting centre in the medulla region of the brain, an important part of which is the chemotrigger zone (CTZ). The vomiting centre possesses neurons which are rich in muscarinic cholinergic and histamine containing synapses. These types of neurons are especially involved in transmission from the vestibular apparatus to the vomiting centre. Motion sickness principally involves overstimulation of these pathways due to various sensory stimuli. Hence the action of cyclizine which acts to block the histamine receptors in the vomiting centre and thus reduce activity along these pathways. Furthermore since cyclizine possesses anti-cholinergic properties as well, the muscarinic receptors are similarly blocked. .../IT SEEMS/ THAT STIMULATION OF VESTIBULAR APPARATUS IS NECESSARY & SUFFICIENT...& THAT VESTIBULAR CEREBELLAR MIDBRAIN "INTEGRATIVE VOMITING CENTER" & MEDULLARY CHEMORECEPTIVE TRIGGER ZONE ARE...INVOLVED /IN MOTION SICKNESS/. IT IS...PROBABLE THAT EFFECTIVE ANTIHISTAMINES EXERT.../ACTION/ IN THESE CENTERS. /ANTIHISTAMINE/ Therapeutic Uses Antiemetics; Histamine H1 Antagonists ANTIHISTAMINE USED AS HYDROCHLORIDE & LACTATE IN PREVENTION & TREATMENT OF MOTION SICKNESS (NAUSEA, VOMITING & VERTIGO). IT IS ALSO PROBABLY EFFECTIVE FOR CONTROL OF POSTOPERATIVE NAUSEA & VOMITING. DURATION OF ACTION IS ABOUT 4 HR. ...LARGE SCALE STUDY, INCL PREGNANT WOMEN RECEIVING CYCLIZINE DURING 1ST TRIMESTER, FAILED TO CONFIRM THAT DRUG HAD ANY TERATOGENIC EFFECT IN MAN WITH DOSES EMPLOYED. For more Therapeutic Uses (Complete) data for CYCLIZINE (7 total), please visit the HSDB record page. Drug Warnings .../DO NOT/ EXCEED 4 TABLETS/DAY. /HYDROCHLORIDE/ .../IT/ SHOULD NOT BE USED IN WOMEN DURING PREGNANCY & THOSE LIKELY TO BECOME PREGNANT UNLESS SPECIFICALLY DIRECTED BY PHYSICIAN. IN 1965 AN AD HOC COMMITTEE OF US FDA CONCLUDED THAT EVIDENCE OF TERATOGENIC EFFECTS IN HUMAN...WAS NOT SIGNIFICANT, BUT IT MADE NO SPECIFIC MENTION OF EYES. ...OFFSPRING OF PROBABLY SEVERAL THOUSAND WOMEN WHO HAD RECEIVED APPROX 150 MG CYCLIZINE/DAY DURING PREGNANCY...HAD A VARIETY OF NONOCULAR ABNORMALITIES, BUT STATISTICAL SIGNIFICANCE IN RELATION TO CYCLIZINE WAS UNCERTAIN. For more Drug Warnings (Complete) data for CYCLIZINE (8 total), please visit the HSDB record page. Pharmacodynamics Cyclizine is a piperazine-derivative antihistamine used as an antivertigo/antiemetic agent. Cyclizine is used in the prevention and treatment of nausea, vomiting, and dizziness associated with motion sickness. Additionally, it has been used in the management of vertigo in diseases affecting the vestibular apparatus. Although the mechanism by which cyclizine exerts its antiemetic and antivertigo effects has not been fully elucidated, its central anticholinergic properties are partially responsible. The drug depresses labyrinth excitability and vestibular stimulation, and it may affect the medullary chemoreceptor trigger zone. It also possesses anticholinergic, antihistaminic, central nervous system depressant, and local anesthetic effects. |
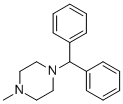
| 分子式 |
C18H22N2
|
|---|---|
| 分子量 |
266.38
|
| 精确质量 |
266.178
|
| CAS号 |
82-92-8
|
| 相关CAS号 |
Cyclizine dihydrochloride;5897-18-7;Cyclizine lactate;5897-19-8;Cyclizine hydrochloride;303-25-3
|
| PubChem CID |
6726
|
| 外观&性状 |
White to off-white solid powder
|
| 熔点 |
105.5-107.5
105.5 TO 107.5 °C |
| LogP |
2.899
|
| tPSA |
6.48
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
20
|
| 分子复杂度/Complexity |
253
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
UVKZSORBKUEBAZ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C18H22N2/c1-19-12-14-20(15-13-19)18(16-8-4-2-5-9-16)17-10-6-3-7-11-17/h2-11,18H,12-15H2,1H3
|
| 化学名 |
1-benzhydryl-4-methylpiperazine
|
| 别名 |
NautazineCYCLIZINE Valoid Neo-devomitMarezine Ciclizina
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~375.40 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (9.39 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (9.39 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (9.39 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7540 mL | 18.7702 mL | 37.5404 mL | |
| 5 mM | 0.7508 mL | 3.7540 mL | 7.5081 mL | |
| 10 mM | 0.3754 mL | 1.8770 mL | 3.7540 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03931135 | UNKNOWN STATUS | Drug: IV Cyclizine versus IV Dexamethasone for prevention of nausea and vomiting after intrathecal morphine in patients undergoing cesarean section |
Spinal Anesthetics Causing Adverse Effects in Therapeutic Use | Assiut University | 2019-09-01 | Not Applicable |
| NCT06186141 | RECRUITING | Drug: Morphine Drug: Oxycodone |
Patient-Controlled Analgesia | Murdoch Childrens Research Institute | 2024-03-13 | Phase 4 |
| NCT01303809 | COMPLETED | Other: Enhanced Recovery After Surgey for Sleeve Gastrectomy | Morbid Obesity | University of Auckland, New Zealand | 2011-05 | Not Applicable |
| NCT02009306 | COMPLETED | Drug: PecFent and Epistatus Drug: Standard subcutaneous medication Drug: Epistatus Alone |
Terminal Cancer | Gloucestershire Hospitals NHS Foundation Trust | 2017-01-23 | Phase 4 |
| NCT06047119 | RECRUITING | Procedure: Blood pressure Procedure: Positive end expiratory pressure Procedure: Tidal volume Procedure: Fraction of inspired oxygen |
Patients Undergoing General Anesthesia | Lars Wiuff Andersen | 2023-10-09 | Not Applicable |