| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Natural product
|
|---|---|
| 体外研究 (In Vitro) |
D-pinitol 刺激 p53 和 Bax,同时抑制 NF-κB 和 Bcl-2,从而诱导 MCF-7 细胞凋亡 [3]。
从天然产物中开发药物一直是一个渐进的过程。许多植物衍生的化合物对各种人类疾病具有极好的治疗潜力。它们是重要的来源,尤其是抗癌剂。基于肿瘤领域的选择性分子靶点,许多有前景的新药正处于临床开发阶段。D-pinitol是一种从大豆中提取的天然化合物,具有重要的药理活性。因此,我们选择D-pinitol来评估MCF-7细胞系的凋亡潜能。用不同浓度的D-pinitol处理人乳腺癌细胞,用MTT和LDH测定细胞毒性。结合p53、Bcl-2、Bax和NF-kB蛋白的表达,研究细胞凋亡的机制。结果显示,D-pinitol显著抑制MCF-7细胞的增殖,并呈浓度依赖性,上调p53、Bax的表达,下调Bcl-2和NF-kB的表达。因此,本研究的结果清楚地证明了D-pinitol通过调节促凋亡和抗凋亡级联蛋白诱导MCF-7细胞凋亡。[3] |
| 体内研究 (In Vivo) |
D-pinitol是一种从松科和豆科植物中分离出来的化合物,据报道具有类似胰岛素的特性。虽然近年来已经认识到D-pinitol的降糖作用,但D-pinitol治疗糖尿病的分子机制尚不清楚。本研究通过饲喂高脂饮食(HFD)并注射链脲佐菌素(STZ)建立2型糖尿病(T2DM)胰岛素抵抗模型,旨在进一步探讨其治疗T2DM的机制。D-pinitol分2次给药[30、60 mg/(kg·体重·天)]。高剂量组大鼠空腹血糖(FBG)水平降低12.63%,d -匹尼醇处理组大鼠口服糖耐量能力提高。生化指标显示D-pinitol对降血糖有积极作用。Western boltting表明D-pinitol可以促进磷脂酰肌醇-3激酶(PI3K) p85、PI3Kp110以及下游靶蛋白激酶B/Akt (Ser473位点)的表达。D-pinitol抑制糖原合成激酶-3β (GSK-3β)蛋白的表达,调节糖原合成(GS)蛋白的表达,从而促进糖原合成。综上所述,D-pinitol在T2DM大鼠中通过易位和激活PI3K/Akt信号通路调节胰岛素介导的肝脏葡萄糖摄取中发挥了积极作用[1]。
D-pinitol是一种环糖醇,存在于几种可食用植物中,被广泛研究用于治疗人类代谢性疾病,作为食物补充剂,并被证明对心血管系统有保护作用。基于这些原因,本研究旨在探讨D-pinitol对小鼠肠系膜动脉血管作用的机制。雄性C57BL/6小鼠肠系膜动脉在钢丝肌图上显示。亚硝酸盐采用2,3-二氨基萘(DAN)法测定。Western blot检测蛋白表达和磷酸化水平。收缩压(SBP)采用尾袖容积描记仪测量。D-pinitol在内皮完好的动脉中诱导浓度依赖性血管扩张,但在内皮脱落的动脉中没有。ω-硝基- l -精氨酸甲酯(300 μM)消除了D-pinitol的作用,1H-[1,2,4]恶二唑[4,3-a]喹诺沙林-1-酮(ODQ;10 μM)使浓度-响应曲线向右偏移。KN-93 (1 μM)减弱了D-pinitol的血管扩张作用,而H-89 (0.1 μM)没有改变D-pinitol的血管扩张作用。1-[2-(三氟甲基)苯基]咪唑(300 μM)、吲哚美辛(10 μM)、塞来昔布(5 μM)、wortmannin (1 μM)、钌红(10 μM)、铁(10 μM)、MnTMPyP (30 μM)、MPP (0.1 μM)、PHTPP (0.1 μM)和阿托品(1 μM)对D-pinitol的作用没有影响。D-pinitol增加了亚硝酸盐的浓度,L-NAME和10 μM卡咪唑抑制了亚硝酸盐的浓度。D-pinitol增加eNOS活化位点Ser1177的磷酸化水平,降低eNOS失活位点Thr495的磷酸化水平。在血压正常的小鼠中,腹腔注射D-pinitol (10 mg/kg)可在30分钟后显著降低收缩压。目前的结果使我们得出结论,D-pinitol在小鼠肠系膜动脉中具有内皮和一氧化氮依赖的血管扩张作用,其机制依赖于钙-钙调蛋白复合物激活eNOS,这可以解释其在小鼠中的降压作用。[2] |
| 酶活实验 |
Pinitol (3- o -甲基-毛肌醇)是传统印度草药(talisapatra)的一种成分,已被证明具有抗炎和抗糖尿病的活性,其机制尚未明确。由于转录因子核因子- kappab (NF-kappaB)与包括胰岛素抵抗在内的炎症性疾病有关,我们假设pinitol必须通过调节NF-kappaB激活途径来调节其作用。我们发现pinitol可以抑制炎症刺激和致癌物诱导的NF-kappaB活化。这种抑制并不局限于细胞类型。除了诱导外,pinitol还可以消除大多数肿瘤细胞中存在的NF-kappaB的组成性激活。pinitol通过抑制ikappabα激酶的激活来抑制NF-kappaB的激活,导致ikappabα磷酸化、ikappabα降解、p65磷酸化、p65核易位和NF-kappaB依赖性报告基因表达的顺序抑制。Pinitol还抑制肿瘤坏死因子受体(TNFR)-1、TNFR相关死亡结构域、TNFR相关因子-2、转化生长因子- β活化激酶-1 (TAK-1)/ tak1结合蛋白-1和ikappabα激酶诱导的NF-kappaB报告细胞活性,但不抑制p65诱导的NF-kappaB报告细胞活性。NF-kappaB活化的抑制导致参与炎症(环氧化酶-2)、增殖(cyclin D1和c-myc)、侵袭(基质金属蛋白酶-9)、血管生成(血管内皮生长因子)和细胞存活(cIAP1、cIAP2、x -连锁抑制细胞凋亡蛋白、Bcl-2和Bcl-xL)的基因产物下调。pinitol抑制这些基因产物可增强TNF和化疗药物诱导的细胞凋亡,抑制TNF诱导的细胞侵袭。我们的研究结果表明,pinitol抑制NF-kappaB激活途径,这可能解释了其抑制炎症细胞反应的能力。[4]
|
| 细胞实验 |
Maintenance of michigan cancer foundation-7 (MCF-7) cell lines/MCF-7 (MCF-7)细胞株的维持[3]
人乳腺癌细胞系MCF-7在加有10% v/v热灭活胎牛血清(FBS)、抗生素(如青霉素50 U/mL、链霉素50µg/mL和1 mmol/L丙酮酸钠)的Dulbecco改良eagle培养基(DMEM)中单层培养,标准条件下,在加5% CO2、37℃的加湿培养箱中培养。培养基每三天更换一次。 Viability assay[3] 采用Mossmann (1983) MTT(34,5 -二甲基噻唑-2-基-2,5-二苯基溴化四氮唑)法测定细胞活力。细胞活力计算公式为:%生长抑制=(处理细胞A570nm /对照细胞A570nm)×100。 |
| 动物实验 |
Vascular Reactivity[2]
Sixty-six male C57BL/6 mice, aged 10–12 weeks, were used in the present study. Mice were euthanized by decapitation, the abdomen was cut, and the mesenteric bed was quickly removed and placed in a dissecting plate with physiological salt solution (PSS) with the following composition (mM): NaCl 119.0; KCl 4.7; KH2PO4 0.4; NaHCO3 14.9; MgSO4.7H2O 1.17; CaCl2.2H2O 2.5; and glucose 5.5. A segment of the second branch of the mesenteric artery was dissected, and the adipose and connective tissues were removed. The arteries were sectioned into rings (1.6–2.0 mm long) with an internal diameter ranging from 150 to 250 μm. The rings were mounted in a wire myograph (620M, DMT, Denmark), kept in carbogen aerated PSS at 37°C. After mounting, the artery was stretched to a length that yielded a circumference equivalent to 90% of that given by an internal pressure of 100 mmHg; this required a load of approximately 200 mg. The vessel was maintained for an equilibration period of 60 min. The mechanical activity was recorded isometrically as previously described (Silva et al., 2016). The functionality of the arteries was observed by the contraction induced by phenylephrine (3 μM) and by the vasodilator effect induced by acetylcholine (ACh, 10 μM) in arteries pre-contracted with phenylephrine. Arteries with ACh-induced vasodilatation higher than 70% were considered with functional endothelium. In some experimental procedures, the endothelium was removed by rubbing the lumen slightly with the tungsten wire. The removal of the endothelium was confirmed by the absence of vasodilatation induced by ACh in precontracted arteries. The vasodilator effect of D-pinitol was evaluated by concentration-response curves (1 nM to 100 μM) in mesenteric arteries in the presence and the absence of a functional endothelium pre-contracted with phenylephrine (3 μM). The participation of nitric oxide synthase (NOS) was investigated in arteries pretreated with Nω-nitro-L-arginine-methyl-ester (L-NAME; 300 μM), a non-selective inhibitor of NOS, and 1-(2-trifluoromethylphenyl) imidazole (TRIM; 300 μM), a selective inhibitor of neuronal NOS (nNOS). The activation of guanylate cyclase was investigated with 1H- [1,2,4]-oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; 10 μM). The involvement of cyclooxygenase (COX) 1 and 2, phosphatidylinositol-3-kinase (PI3K), Ca2+/calmodulin-dependent kinase II (CaMKII), and non-selective cationic channels was verified in arteries pretreated with indomethacin (10 μM), celecoxib (5 μM), wortmannin (1 μM), KN-93 (1 μM), and ruthenium red (10 μM), respectively. Tiron (10 μM) and MnTMPyP (30 μM), a cell-permeable analog of superoxide dismutase, were used to investigate the action of antioxidant drugs on the vasodilator effect of D-pinitol. The participation of muscarinic receptors and α and β estrogen receptors was investigated in arteries pretreated with atropine (1 μM), MPP (0.1 μM), and PHTPP (0.1 μM), respectively. Nitrite Measurement in Mouse Mesenteric Artery[2] The assessment of NO production in the mesenteric artery was performed indirectly by the measurement of nitrite (NO2-) using the fluorescence method with 2,3-diaminonaphthalene (DAN), according to Silva et al. (2016). The mesenteric artery branches were placed in PSS, at 37°C in 5% CO2 atmosphere. Samples were collected in the absence (basal) or the presence of D-pinitol (20 μM) or ACh (10 μM). The involvement of NOS and calmodulin in the production of nitrite was evaluated in the presence of L-NAME (300 μM) and calmidazolium (10 μM), respectively. 150 μl samples were collected, added to 150 μl of purified water, followed by the immediate addition of 15 μl fresh DAN solution (0.05 mg/l in 0.62 M HCl) in 96-well opaque black plates (Costar®, United States). The reaction proceeded for 10 min at room temperature and protected from light. After this period, the reaction was stopped with 5 μl of NaOH (2.8 N) and the absorbance determined using a spectrofluorometer (Fluoroskan Ascent FL, Thermo Scientific) at 365 and 415 nm, as respective excitation and emission wavelengths. The nitrite concentration in the samples was calculated using a standard curve with predetermined concentrations of sodium nitrite in each experiment and normalized by the amount of protein in the branches. The results were expressed in [nitrite] nM/μg of protein. |
| 参考文献 |
|
| 其他信息 |
D-pinitol is the D-enantiomer of pinitol. It has a role as a geroprotector and a member of compatible osmolytes. It is functionally related to a 1D-chiro-inositol. It is an enantiomer of a L-pinitol.
Methylinositol has been used in trials studying the treatment of Dementia and Alzheimer's Disease. D-Pinitol has been reported in Abies pindrow, Glycine max, and other organisms with data available. See also: Ononitol, (+)- (annotation moved to). |
| 分子式 |
C7H14O6
|
|---|---|
| 分子量 |
194.1825
|
| 精确质量 |
194.079
|
| 元素分析 |
C, 43.30; H, 7.27; O, 49.43
|
| CAS号 |
10284-63-6
|
| PubChem CID |
164619
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.6±0.1 g/cm3
|
| 沸点 |
317.2±42.0 °C at 760 mmHg
|
| 熔点 |
178-185ºC
|
| 闪点 |
145.6±27.9 °C
|
| 蒸汽压 |
0.0±1.5 mmHg at 25°C
|
| 折射率 |
1.588
|
| LogP |
-0.74
|
| tPSA |
110.38
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
13
|
| 分子复杂度/Complexity |
158
|
| 定义原子立体中心数目 |
4
|
| SMILES |
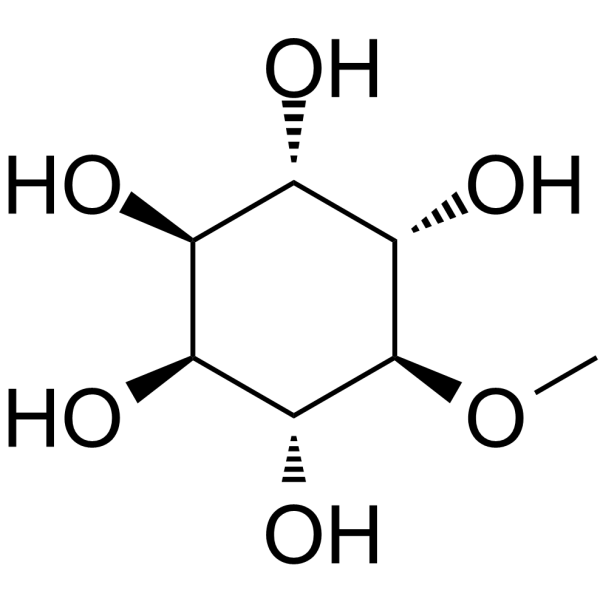
COC1[C@@H]([C@H](C([C@@H]([C@@H]1O)O)O)O)O
|
| InChi Key |
DSCFFEYYQKSRSV-KLJZZCKASA-N
|
| InChi Code |
InChI=1S/C7H14O6/c1-13-7-5(11)3(9)2(8)4(10)6(7)12/h2-12H,1H3/t2-,3-,4-,5-,6+,7+/m0/s1
|
| 化学名 |
3-O-methyl-D-chiro-inositol
|
| 别名 |
10284-63-6; Pinitol; 3-O-Methyl-D-chiro-inositol; (+)-Pinitol; Inzitol; D-ononitol; Methylinositol;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~125 mg/mL (~643.73 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (10.71 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (10.71 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (10.71 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.1499 mL | 25.7493 mL | 51.4986 mL | |
| 5 mM | 1.0300 mL | 5.1499 mL | 10.2997 mL | |
| 10 mM | 0.5150 mL | 2.5749 mL | 5.1499 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。