| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
|

| 靶点 |
5-HT
|
|---|---|
| 体外研究 (In Vitro) |
度洛西汀((S)-盐酸度洛西汀)(以商品名 Cymbalta、Ariclaim、Xeristar、Yentreve、Duzela、Dulane 销售)是一种 5-羟色胺-去甲肾上腺素再摄取抑制剂 (SNRI),由礼来公司生产和销售。它适用于治疗重度抑郁症和广泛性焦虑症(GAD)。度洛西汀还被批准用于治疗骨关节炎和肌肉骨骼疼痛。由于担心肝毒性和自杀事件,度洛西汀未能在美国批准用于治疗压力性尿失禁;然而,它在欧洲被批准用于该适应症,并被推荐作为压力性尿失禁的附加药物而不是手术。它还可以缓解疼痛性周围神经病变的症状,特别是糖尿病性神经病变,并用于控制纤维肌痛的症状。度洛西汀的主要用途是治疗重度抑郁症、广泛性焦虑症、压力性尿失禁、疼痛性周围神经病、纤维肌痛以及与骨关节炎和慢性下背痛相关的慢性肌肉骨骼疼痛。正在研究它的各种其他适应症[1][2]。
|
| 体内研究 (In Vivo) |
奥沙利铂是一种广泛使用的化疗药物,但会引起严重的周围神经病变。度洛西汀是血清素和去甲肾上腺素的双重再摄取抑制剂,已被证明对疼痛有效。然而,度洛西汀是否以及如何减轻奥沙利铂诱导的啮齿动物异常性疼痛尚不清楚。单次注射奥沙利铂(6mg/kg,腹腔注射;i.p.)可引起冷性和机械性异常性疼痛,分别通过丙酮和von Frey细丝试验进行评估。当观察到明显的异常性疼痛症状时,注射了三种不同剂量的度洛西汀(10、30和60mg/kg,i.p.)。给予30和60mg/kg度洛西汀可显著减轻异常性疼痛,而10mg/kg则没有。通过使用体内细胞外记录方法,我们进一步证实30mg/kg的度洛西汀可以显著抑制脊髓宽动态范围(WDR)细胞的高兴奋性。鞘内注射酚妥拉明(非选择性α-肾上腺素能受体拮抗剂,20μg)或哌唑嗪(α₁-肾上腺素能拮抗剂,10μg)可完全阻断度洛西汀的抗异常性疼痛作用;然而,咪达唑嗪(α8322-肾上腺素能受体拮抗剂,10μg)没有阻断它。总之,我们认为度洛西汀可能对奥沙利铂诱导的神经性疼痛和脊髓过度兴奋具有有效的保护作用,这是由脊髓α8321-肾上腺素能受体介导的[2]。
|
| 酶活实验 |
乳酸脱氢酶测定[1]
乳酸脱氢酶(LDH)是一种细胞内酶,在细胞死亡过程中释放到上清液中。根据制造商的说明,使用LDH诊断试剂盒测量细胞膜损伤后释放到培养基中的LDH。每组重复三次进行统计分析。[1] 脂质过氧化试验[1] 通过测量丙二醛(MDA)的浓度来确定脂质过氧化水平,丙二醛是脂质过氧化的最终产物,与TBA反应形成荧光加合物。根据制造商的说明,使用脂质过氧化MDA测定试剂盒测定总MDA量。使用Pierce BCA蛋白测定试剂盒测定总蛋白含量。各组的MDA水平为总MDA除以总蛋白。每组重复三次进行统计分析。 |
| 细胞实验 |
细胞活力测定[1]
细胞以每孔2×105个细胞的密度接种在96孔板中,生长24小时,然后根据时间依赖性或剂量依赖性方案用药物处理。每种治疗均进行三次。药物处理后,根据制造商的说明,使用细胞计数试剂盒-8(CCK-8)测定细胞存活率。CCK-8使用灵敏的比色WST-8测定法来确定活细胞的数量。WST-8是一种高度水溶性的四唑盐,化学名称为2-(2-甲氧基-4-硝基苯基)-3-(4-硝基苯基)-5-(2,4-二磺苯基)-2H-四唑单钠盐。[1] 菌落形成试验[1] N2a细胞用各种指定浓度的度洛西汀处理24小时。含有1×103个细胞的6孔板的三孔孔用不同浓度的度洛西汀处理,并再维持21天。用甲醇固定菌落,在室温下用0.1%结晶紫溶液染色1小时,并计数。菌落形成试验重复三次。[1] N2a细胞分化[1] N2a细胞通过涉及RA添加和血清撤回的方案进行分化。分化培养基为DMEM,补充20μM RA、100 U/ml青霉素和100μg/ml链霉素。神经突起被鉴定为长度大于两个细胞体直径的细胞突起。分化的细胞被定义为具有神经突起的细胞。通过在每个孔的六个随机选择的区域中计数180个细胞来对百分比进行统计分析。神经突起长度定义为从细胞体到神经突起尖端的距离。使用ImageJ软件在五个随机选择的区域中的至少50个细胞中测量了最长神经突起的长度。为了评估细胞毒性,将致力于与RA分化的N2a细胞加入12.5μM度洛西汀或12.5μM德洛西汀加10μM利福平处理24小时。在处理24小时后,对对照组、RA组、RA+度洛西丁组和RA+度洛西汀+利福平组的细胞形态进行了统计比较(n=3)。在完全分化期间(n=3),每天记录细胞存活率和细胞形态。此外,在对照组和RA组的不同时间点分析了与细胞周期和细胞死亡相关的事件,以解释N2a细胞分化过程中的关键变化。在24小时治疗后,对对照组、RA组、RA+度洛西汀组的细胞死亡(n=4)、细胞周期(=4)和生化变化(n=3)进行了统计分析。 |
| 动物实验 |
Duloxetine and α-Adrenergic Receptor Antagonists Administration[2]
Duloxetine was dissolved in distilled water (D.W.). Different doses of duloxetine (10, 30, and 60 mg/kg) were administered (i.p.). To test which adrenergic receptor subtypes mediated the anti-allodynic effects of duloxetine in oxaliplatin-administered mice, antagonists were administered intrathecally 20 min prior to duloxetine treatments. Non-selective α-adrenergic antagonists (phentolamine, 20 μg), α1-adrenergic receptor antagonists (prazosin, 10 μg), and α2-adrenergic receptor antagonists (idazoxan, 10 μg) were administered in volumes of 5 μL. The dose of each antagonist was determined based on previously conducted studies showing the selective and effective antagonistic action against adrenergic receptor-mediated responses.[2] In Vivo Extracellular Recording[2] Extracellular recordings were made from Sprague-Dawley rats, three to five days after the administration of oxaliplatin, when rats exhibited significant mechanical and cold allodynia. Extracellular recordings were carried out as previously described. In brief, rats were anesthetized with urethane (1.5 g/kg, i.p.). The spinal cords of the animals, which were fixed in a stereotaxic frame, was exposed from T13–L2 and irrigated with oxygenated (95% O2-5% CO2 gas) Krebs solution (in mM: 117 NaCl, 3.6 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 11 glucose, and 25 NaHCO3) at a flow rate of 10–15 mL/min at 38 ± 1 °C. Based on their responses to brush, pressure, pinch, and acetone stimulations, the WDR cells were classified. Extracellular single-unit recordings were made with a low-impedance insulated tungsten microelectrode (impedance of 10 MΩ). For mechanical stimuli, brush, press, and pinch stimulations were applied to the lateral and ventral surfaces of the hind paw. Brush stimulus was given by brushing the receptive field five times with a camel brush. Press stimulus was given by pressing the receptive field for 4 s using the blunt tip of the camel brush with a diameter of 0.5 cm and a magnitude of about 20 g. Pinch stimulation was given by pinching the skin using toothed forceps for 3 s. For cold stimulation, 10 μL of acetone drop was applied to the receptive fields. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Little published information is available on the use of duloxetine during breastfeeding; however, the dose in milk is low and serum levels were low in two breastfed infants. If the mother requires duloxetine, it is not a reason to discontinue breastfeeding. Expert opinion finds duloxetine acceptable to use during breastfeeding, and a safety scoring system finds duloxetine use to be possible to use cautiously during breastfeeding. An alternate drug that has been better studied may be preferred, especially while nursing a newborn or preterm infant. Monitor the infant for drowsiness and adequate feeding, weight gain and developmental milestones, especially in younger, exclusively breastfed infants and when using combinations of psychotropic drugs. Galactorrhea has been reported in women taking duloxetine. ◉ Effects in Breastfed Infants A partially nursing mother was taking duloxetine 90 mg and extended-release methylphenidate (Concerta) 36 mg daily for ADHD, generalized anxiety disorder, borderline personality disorder, and depression. She partially (amount not stated) breastfed her infant for about 1 month. At 6 months of age, infant's development was considered to be normal, except for recurrent pneumonia caused by congenital pulmonary airway malformation. Another mother took duloxetine 60 mg daily while partially (amount not stated) nursing her infant. At 6 weeks of age, no adverse events were observed in the exposed infant. One mother reported a possible adverse reaction of drowsiness in her baby in the first few weeks after birth. She was taking agomelatine in an unspecified dose with duloxetine 90 mg daily. She attributed the drowsiness to agomelatine and continued breastfeeding her baby until 9 months of age. She reported some developmental concerns of speech and low muscle tone in her baby who was 9 months of age at the time of follow-up. ◉ Effects on Lactation and Breastmilk In a small prospective study, 8 primiparous women who were taking a serotonin reuptake inhibitor (SRI; 3 taking fluoxetine and 1 each taking citalopram, duloxetine, escitalopram, paroxetine or sertraline) were compared to 423 mothers who were not taking an SRI. Mothers taking an SRI had an onset of milk secretory activation (lactogenesis II) that was delayed by an average of 16.7 hours compared to controls (85.8 hours postpartum in the SRI-treated mothers and 69.1 h in the untreated mothers), which doubled the risk of delayed feeding behavior compared to the untreated group. However, the delay in lactogenesis II may not be clinically important, since there was no statistically significant difference between the groups in the percentage of mothers experiencing feeding difficulties after day 4 postpartum. After one nonpregnant woman began taking duloxetine, her serum prolactin increased and previous galactorrhea, which had decreased after stopping venlafaxine, increased again. After stopping duloxetine, her prolactin decreased to normal and galactorrhea ceased. A woman who was taking duloxetine at an unspecified dose for depression reported a milky discharge from her nipples. She had not experienced this effect with previous antidepressant therapy. Her serum prolactin was elevated, and an MRI of her head found no tumors. Duloxetine was stopped and she was treated with escitalopram 20 mg daily and cabergoline 0.5 mg twice weekly for one month. At this time her serum prolactin was normal and the galactorrhea had stopped. In a study of cases of hyperprolactinemia and its symptoms (e.g., gynecomastia) reported to a French pharmacovigilance center, duloxetine was not found to have an increased risk of causing hyperprolactinemia compared to other drugs. A woman taking duloxetine 60 mg daily for depression complained of a milky breast discharge, breast fullness, and breast pain, after taking the drug for a total of 10 weeks. Duloxetine was discontinued and bupropion was started. Two weeks after stopping duloxetine, galactorrhea improved. Six weeks after stopping duloxetine, her serum prolactin had dropped from the previous level of 37.9 mcg/L to 20.2 mcg/L. Her galactorrhea was probably caused by duloxetine. A woman being treated for migraine with duloxetine 30 mg daily began to have bilateral galactorrhea during the tenth week of treatment. At that time and on repeated measurements, her serum prolactin level was within the normal range. Her galactorrhea ceased 3 days after discontinuation of duloxetine. The authors found that her galactorrhea was probably caused by duloxetine. An observational study looked at outcomes of 2859 women who took an antidepressant during the 2 years prior to pregnancy. Compared to women who did not take an antidepressant during pregnancy, mothers who took an antidepressant during all 3 trimesters of pregnancy were 37% less likely to be breastfeeding upon hospital discharge. Mothers who took an antidepressant only during the third trimester were 75% less likely to be breastfeeding at discharge. Those who took an antidepressant only during the first and second trimesters did not have a reduced likelihood of breastfeeding at discharge. The antidepressants used by the mothers were not specified. A retrospective cohort study of hospital electronic medical records from 2001 to 2008 compared women who had been dispensed an antidepressant during late gestation (n = 575) to those who had a psychiatric illness but did not receive an antidepressant (n = 1552) and mothers who did not have a psychiatric diagnosis (n = 30,535). Women who received an antidepressant were 37% less likely to be breastfeeding at discharge than women without a psychiatric diagnosis, but no less likely to be breastfeeding than untreated mothers with a psychiatric diagnosis. None of the mothers were taking duloxetine. A woman with major depressive disorder received duloxetine 40 mg twice daily. After 2 weeks, she developed menstrual irregularities and a milky discharge from her breasts. Her serum prolactin was elevated at 205 mcg/L. The duloxetine dosage was decreased to 60 mg once daily and aripiprazole was begun at 2.5 mg daily and then increased to 5 mg daily. Within 2 weeks, galactorrhea had stopped and the serum prolactin had decreased to 118 mcg/L. Six weeks later, serum prolactin was 39 mcg/L. The combination was continued for another 39 weeks with no return of galactorrhea. A 16-year-old girl was admitted for depression and suicide attempts. She had previously experienced galactorrhea while taking risperidone and escitalopram. She was started on duloxetine 20 mg daily which was increased to 40 mg daily after 5 days. Two days later, small amounts of milk appeared from the right breast. Her serum prolactin was mildly elevated at 26 mcg/L. The dose was reduced to 20 mg daily and the milk production ceased. A woman with depression was treated with duloxetine 30 mg daily for 1 month, then 60 mg daily. After 4 months of therapy she presented with amenorrhea, lactation and hyperprolactinemia. The patient was treated with cabergoline 0.5 mg twice weekly and duloxetine was discontinued. One month later, the serum prolactin level was normal. A woman with multiple sclerosis had been treated with duloxetine 60 mg daily for pain and depression for 3 months. She noted a milk-like breast discharge for a month and her serum prolactin was elevated. Duloxetine was changed to escitalopram 10 mg daily. Within days, her galactorrhea stopped and her serum prolactin decreased. Cabergoline 0.25 mg twice weekly was instituted after other causes of hyperprolactinemia were ruled out. The dose was reduced after 3 months and her serum prolactin remained normal. In a study of 80,882 Norwegian mother-infant pairs from 1999 to 2008, new postpartum antidepressant use was reported by 392 women and 201 reported that they continued antidepressants from pregnancy. Compared with the unexposed comparison group, late pregnancy antidepressant use was associated with a 7% reduced likelihood of breastfeeding initiation, but with no effect on breastfeeding duration or exclusivity. Compared with the unexposed comparison group, new or restarted antidepressant use was associated with a 63% reduced likelihood of predominant, and a 51% reduced likelihood of any breastfeeding at 6 months, as well as a 2.6-fold increased risk of abrupt breastfeeding discontinuation. Specific antidepressants were not mentioned. |
| 参考文献 |
|
| 其他信息 |
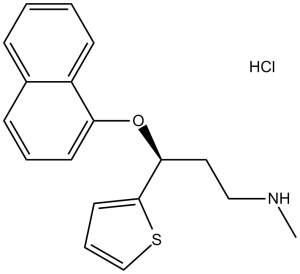
(S)-duloxetine hydrochloride is a duloxetine hydrochloride in which the duloxetine moiety has S configuration. It has a role as an antidepressant. It contains a (S)-duloxetine.
Duloxetine Hydrochloride is the hydrochloride salt of duloxetine, an orally bioavailable fluoxetine derivative belonging to the class of selective serotonin (5-HT) and norepinephrine (NE) reuptake inhibitors (SNRIs), with central pain inhibitory, anxiolytic and antidepressant activities. Upon oral administration, duloxetine selectively prevents the reuptake of 5-HT and NE via transporter complexes on the pre-synaptic membrane, thereby increasing the level of these neurotransmitters within the synaptic cleft. This potentiates serotonergic and noradrenergic activities in the central nervous system (CNS), and alleviates anxiety, depression, and neuropathy sensations, such as neuropathic pain. A thiophene derivative and selective NEUROTRANSMITTER UPTAKE INHIBITOR for SEROTONIN and NORADRENALINE (SNRI). It is an ANTIDEPRESSIVE AGENT and ANXIOLYTIC, and is also used for the treatment of pain in patients with DIABETES MELLITUS and FIBROMYALGIA. See also: Duloxetine (has active moiety). Drug Indication Treatment of major depressive disorder; Treatment of diabetic peripheral neuropathic pain; Treatment of generalised anxiety disorder; Duloxetine Mylan is indicated in adults. Treatment of major depressive disorder. Treatment of diabetic peripheral neuropathic pain. Treatment of generalised anxiety disorder. Cymbalta is indicated in adults. Yentreve is indicated for women for the treatment of moderate to severe stress urinary incontinence (SUI). Duloxetine Lilly is indicated in adults for: Treatment of major depressive disorderTreatment of diabetic peripheral neuropathic painTreatment of generalised anxiety disorderDuloxetine Lilly is indicated in adults. Treatment of diabetic peripheral neuropathic pain. Ariclaim is indicated in adults. Treatment of diabetic peripheral neuropathic pain in adults. Treatment of chronic pain, Treatment of diabetic neuropathic pain, Treatment of generalised anxiety disorder, Treatment of major depressive disorder, Treatment of stress urinary incontinence |
| 分子式 |
C18H20CLNOS
|
|
|---|---|---|
| 分子量 |
333.88
|
|
| 精确质量 |
333.095
|
|
| 元素分析 |
C, 64.75; H, 6.04; Cl, 10.62; N, 4.20; O, 4.79; S, 9.60
|
|
| CAS号 |
136434-34-9
|
|
| 相关CAS号 |
Duloxetine; 116539-59-4; Duloxetine-d3 hydrochloride; 1435727-97-1; (±)-Duloxetine hydrochloride; 947316-47-4; Duloxetine metabolite Para-Naphthol Duloxetine; 949095-98-1
|
|
| PubChem CID |
60834
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 沸点 |
466.2ºC at 760 mmHg
|
|
| 熔点 |
118-122ºC
|
|
| 闪点 |
235.7ºC
|
|
| LogP |
5.823
|
|
| tPSA |
49.5
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
6
|
|
| 重原子数目 |
22
|
|
| 分子复杂度/Complexity |
312
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
Cl[H].S1C([H])=C([H])C([H])=C1[C@]([H])(C([H])([H])C([H])([H])N([H])C([H])([H])[H])OC1=C([H])C([H])=C([H])C2=C([H])C([H])=C([H])C([H])=C12
|
|
| InChi Key |
BFFSMCNJSOPUAY-LMOVPXPDSA-N
|
|
| InChi Code |
InChI=1S/C18H19NOS.ClH/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16;/h2-10,13,17,19H,11-12H2,1H3;1H/t17-;/m0./s1
|
|
| 化学名 |
(3S)-N-methyl-3-naphthalen-1-yloxy-3-thiophen-2-ylpropan-1-amine;hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.49 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.49 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (7.49 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 2% DMSO +30% PEG 300 +ddH2O: 30mg/mL 配方 5 中的溶解度: 140 mg/mL (419.31 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9951 mL | 14.9754 mL | 29.9509 mL | |
| 5 mM | 0.5990 mL | 2.9951 mL | 5.9902 mL | |
| 10 mM | 0.2995 mL | 1.4975 mL | 2.9951 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05267873 | Active Recruiting |
Drug: Duloxetine, Vortioxetine | Depressive Disorder, Major | Johns Hopkins Bloomberg School of Public Health |
January 1, 2015 | N/A |
| NCT05930912 | Active Recruiting |
Drug: sertraline 50mg Drug: Duloxetine 20 MG |
PTSD OCD |
Yang I. Pachankis, PhD | June 1, 2023 | N/A |
| NCT05550506 | Recruiting | Drug: Duloxetine | Chronic Pain Fibromyalgia |
Cukurova University | July 27, 2022 | N/A |
| NCT05611749 | Not yet recruiting | Drug: Duloxetine 60 MG Other: Placebo |
Narcotic Use Opioid Use |
Scripps Health | November 15, 2022 | Phase 2 |
| NCT05311774 | Not yet recruiting | Drug: Duloxetine 30 mg Drug: Tramadol |
Cancer Pain | Assiut University | April 2022 | Not Applicable |