| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|

| 靶点 |
serotonin reuptake; norepinephrine reuptake
|
|---|---|
| 体外研究 (In Vitro) |
度洛西汀((S)-度洛西汀)抑制中枢神经系统中血清素和去甲肾上腺素的再摄取。度洛西汀也被认为是一种效力较弱的多巴胺再摄取抑制剂。然而,度洛西汀对多巴胺能、肾上腺素能、胆碱能、组胺能、阿片类、谷氨酸和 GABA 受体没有显着的亲和力,因此可以被认为是 5-HT 和 NA 转运蛋白的选择性再摄取抑制剂。度洛西汀经历广泛的代谢,但主要的循环代谢物对药理活性没有显着贡献。据信,重度抑郁症部分是由于中枢神经系统内促炎细胞因子的增加所致。抗抑郁药,包括与度洛西汀作用机制相似的抗抑郁药,即抑制血清素代谢,会导致促炎细胞因子活性降低和抗炎细胞因子增加;这种机制可能适用于度洛西汀对抑郁症的作用,但缺乏对度洛西汀治疗特异性细胞因子的研究[1]。度洛西汀在治疗糖尿病神经病变和纤维肌痛等中枢性疼痛综合征中的镇痛特性被认为是由于钠离子通道阻断所致[2]。
|
| 体内研究 (In Vivo) |
给药后约6小时,度洛西汀的最大血浆浓度(Cmax)范围为约47ng/mL(40mg每日两次剂量)至110ng/mL(80mg每日两次剂量)。度洛西汀的消除半衰期约为 10-12 小时,分布体积约为 1640 L。在一项研究中,单剂量 60 mg 后,绝对口服生物利用度平均为 30% 至 80%,平均为 19% 至 19%。另一项平均为 71%。度洛西汀的吸收受食物和一天中时间的影响;食物和睡前给药会导致 4 小时 tmax 延迟[1]。
|
| 细胞实验 |
细胞活力测定[2]
细胞以每孔2×105个细胞的密度接种在96孔板中,生长24小时,然后根据时间依赖性或剂量依赖性方案用药物处理。每种治疗均进行三次。药物处理后,根据制造商的说明,使用细胞计数试剂盒-8(CCK-8)测定细胞存活率。CCK-8使用灵敏的比色WST-8测定法来确定活细胞的数量。WST-8是一种高度水溶性的四唑盐,化学名称为2-(2-甲氧基-4-硝基苯基)-3-(4-硝基苯基)-5-(2,4-二磺苯基)-2H-四唑单钠盐。
|
| 动物实验 |
Duloxetine and α-Adrenergic Receptor Antagonists Administration[3]
Duloxetine was dissolved in distilled water (D.W.). Different doses of duloxetine (10, 30, and 60 mg/kg) were administered (i.p.). To test which adrenergic receptor subtypes mediated the anti-allodynic effects of duloxetine in oxaliplatin-administered mice, antagonists were administered intrathecally 20 min prior to duloxetine treatments. Non-selective α-adrenergic antagonists (phentolamine, 20 μg), α1-adrenergic receptor antagonists (prazosin, 10 μg), and α2-adrenergic receptor antagonists (idazoxan, 10 μg) were administered in volumes of 5 μL. The dose of each antagonist was determined based on previously conducted studies showing the selective and effective antagonistic action against adrenergic receptor-mediated responses.[3]
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Duloxetine is incompletely absorbed with a mean bioavailability of 50% although there is wide variability in the range of 30-80%. The population absorption constant (ka) is 0.168 h-1.The molecule is susceptible to hydrolysis in acidic environments necessitating the use of an enteric coating to protect it during transit through the stomach. This creates a 2 hour lag time from administration to the start of absorption. The Tmax is 6 hours including the lag time. Administering duloxetine with food 3 hour delay in Tmax along with an 10% decrease in AUC. Similarly, administering the dose at bedtime produces a 4 hour delay and 18% decrease in AUC with a 29% reduction in Cmax. These are attributed to delayed gastric emptying in both cases but are not expected to impact therapy to a clinically significant degree. About 70% of duloxetine is excreted in the urine mainly as conjugated metabolites. Another 20% is present in the feces as the parent drug, 4-hydroxy metabolite, and an uncharacterized metabolite. Biliary secretion is thought to play a role due to timeline of fecal excretion exceeding the time expected of normal GI transit. Apparent Vd of 1620-1800 L. Duloxetine crosses the blood-brain barrier and collects in the cerebral cortex at a higher concentration than the plasma. There is a large degree of interindividual variation reported in the clearance of duloxetine with values ranging from 57-114 L/h. Steady state concentrations have still been shown to be dose proportional with a doubling of dose from 30 to 60 mg and from 60 to 120 mg producing 2.3 and 2.6 times the Css respectively. Many additional metabolites have been identified in urine, some representing only minor pathways of elimination. Only trace (<1% of the dose) amounts of unchanged duloxetine are present in the urine. Most (about 70%) of the duloxetine dose appears in the urine as metabolites of duloxetine; about 20% is excreted in the feces. Duloxetine undergoes extensive metabolism, but the major circulating metabolites have not been shown to contribute significantly to the pharmacologic activity of duloxetine. Duloxetine has an elimination half-life of about 12 hours (range 8 to 17 hours) and its pharmacokinetics are dose proportional over the therapeutic range. Steady-state plasma concentrations are typically achieved after 3 days of dosing. Elimination of duloxetine is mainly through hepatic metabolism involving two P450 isozymes, CYP1A2 and CYP2D6. Orally administered duloxetine hydrochloride is well absorbed. There is a median 2 hour lag until absorption begins (Tlag), with maximal plasma concentrations (Cmax) of duloxetine occurring 6 hours post dose. Food does not affect the Cmax of duloxetine, but delays the time to reach peak concentration from 6 to 10 hours and it marginally decreases the extent of absorption (AUC) by about 10%. There is a 3 hour delay in absorption and a one-third increase in apparent clearance of duloxetine after an evening dose as compared to a morning dose. The apparent volume of distribution averages about 1640 L. Duloxetine is highly bound (>90%) to proteins in human plasma, binding primarily to albumin and a1-acid glycoprotein. The interaction between duloxetine and other highly protein bound drugs has not been fully evaluated. Plasma protein binding of duloxetine is not affected by renal or hepatic impairment. Metabolism / Metabolites Duloxetine is extensively metabolized primarily by CYP1A2 and CYP2D6 with the former being the greater contributor. It is hydroxylated at the 4, 5, or 6 positions on the naphthalene ring with the 4-hydroxy metabolite proceeding directly to a glucuronide conjugate while the 5 and 6-hydroxy metabolites proceed through a catechol and a 5-hydroxy, 6-methoxy intermediate before undergoing glucuronide or sulfate conjugation. CYP2C9 is known to be a minor contributor to the 5-hydroxy metabolite. Another uncharacterized metabolite is known to be excreted in the feces but comprises <5% of the total excreted drug. Many other metabolites exist but have not been identified due their low contribution to the overall profile of duloxetine and lack of clinical significance. Biotransformation and disposition of duloxetine in humans have been determined following oral administration of (14C)-labeled duloxetine. Duloxetine comprises about 3% of the total radiolabeled material in the plasma, indicating that it undergoes extensive metabolism to numerous metabolites. The major biotransformation pathways for duloxetine involve oxidation of the naphthyl ring followed by conjugation and further oxidation. Both CYP1A2 and CYP2D6 catalyze the oxidation of the naphthyl ring in vitro. Metabolites found in plasma include 4-hydroxy duloxetine glucuronide and 5-hydroxy, 6-methoxy duloxetine sulfate. Duloxetine has known human metabolites that include 5-((S)-3-Methylamino-1-thiophen-2-yl-propoxy)-naphthalen-2-ol, 5-Hydroxyduloxetine, and 4-Hydroxyduloxetine. The major biotransformation pathways for duloxetine involve oxidation of the naphthyl ring followed by conjugation and further oxidation. Both CYP2D6 and CYP1A2 catalyze the oxidation of the naphthyl ring in vitro. Metabolites found in plasma include 4-hydroxy duloxetine glucuronide and 5-hydroxy, 6-methoxy duloxetine sulfate. The major circulating metabolites have not been shown to contribute significantly to the pharmacologic activity of duloxetine. Route of Elimination: Many additional metabolites have been identified in urine, some representing only minor pathways of elimination. Most (about 70%) of the duloxetine dose appears in the urine as metabolites of duloxetine; about 20% is excreted in the feces. Half Life: 12 hours (range 8-17 hours) Biological Half-Life Mean of 12 h with a range of 8-17. Duloxetine has an elimination half-life of about 12 hours (range 8 to 17 hours) and its pharmacokinetics are dose proportional over the therapeutic range. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Duloxetine hydrochloride is used for the acute management of generalized anxiety disorder in adults, the management of neuropathic pain associated with diabetic peripheral neuropathy in adults, the management of fibromyalgia in adults, the management of moderate to severe stress urinary incontinence (SUI) in women, and the acute and maintenance treatment of major depressive disorder in adults. HUMAN EXPOSURE AND TOXICITY: Possible risk of severe hepatic toxicity; elevated serum transaminase concentrations, sometimes requiring discontinuance of duloxetine, have been reported. In postmarketing experience, fatal outcomes have been reported for acute overdoses, primarily with mixed overdoses, but also with duloxetine only, at doses as low as 1000 mg. Signs and symptoms of overdose (duloxetine alone or with mixed drugs) included somnolence, coma, serotonin syndrome, seizures, syncope, tachycardia, hypotension, hypertension, and vomiting. A higher proportion of patients reporting discontinuation-emergent adverse events were seen with 120 mg/day duloxetine compared with lower doses. For doses between 40 and 120 mg/day duloxetine the proportion of patients reporting at least one discontinuation-emergent adverse event differed significantly from placebo. Extended treatment with duloxetine beyond 8-9 weeks did not appear to be associated with an increased incidence or severity of discontinuation-emergent adverse events. Abrupt discontinuation of duloxetine is associated with a discontinuation-emergent adverse event profile similar to that seen with other selective serotonin reuptake inhibitor (SSRI) and selective serotonin and norepinephrine reuptake inhibitor (SNRI) antidepressants ANIMAL STUDIES: Duloxetine was administered in the diet to mice for 2 years. In female mice receiving duloxetine at 140 mg/kg/day (6 times the maximum recommended human dose (MRHD) of 120 mg/day on a mg/ sq m basis), there was an increased incidence of hepatocellular adenomas and carcinomas. The no-effect dose was 50 mg/kg/day (2 times the MRHD). Tumor incidence was not increased in male mice receiving duloxetine at doses up to 100 mg/kg/day (4 times the MRHD). Duloxetine administered orally to either male or female rats prior to and throughout mating at doses up to 45 mg/kg/day (4 times the MRHD) did not alter mating or fertility. When duloxetine was administered orally to pregnant rats throughout gestation and lactation, the survival of pups to 1 day postpartum and pup body weights at birth and during the lactation period were decreased at a dose of 30 mg/kg/day (5 times the MRHD and 2 times the human dose of 120 mg/day on a mg/sq m basis); the no-effect dose was 10 mg/kg/day. Furthermore, behaviors consistent with increased reactivity, such as increased startle response to noise and decreased habituation of locomotor activity, were observed in pups following maternal exposure to 30 mg/kg/day. Post-weaning growth and reproductive performance of the progeny were not affected adversely by maternal duloxetine treatment. Duloxetine was not mutagenic in the bacterial reverse mutation assay (Ames test), and not clastogenic in an in vivo chromosomal aberration test in mouse bone marrow cells. Additionally, it was not genotoxic in an in vitro mammalian forward gene mutation assay in mouse lymphoma cells or in an in vitro unscheduled DNA assay in rat hepatocytes, and did not induce in vivo sister chromatid exchange assay in Chinese hamster bone marrow cells. Duloxetine is a potent inhibitor of neuronal serotonin and norepinephrine reuptake and a less potent inhibitor of dopamine reuptake. Duloxetine has no significant affinity for dopaminergic, adrenergic, cholinergic, histaminergic, opioid, glutamate, and GABA receptors. The antidepressant and pain inhibitory actions of duloxetine are believed to be related to its potentiation of serotonergic and noradrenergic activity in the CNS. The mechanism of action of duloxetine in SUI has not been determined, but is thought to be associated with the potentiation of serotonin and norepinephrine activity in the spinal cord, which increases urethral closure forces and thereby reduces involuntary urine loss. Toxicity Data Oral, rat LD50: 491 mg/kg for males and 279 mg/kg for females (A308). Interactions Duloxetine is an inhibitor of the CYP1A2 isoform in in vitro studies, and in two clinical studies the average (90% confidence interval) increase in theophylline AUC was 7% (1%-15%) and 20% (13%-27%) when co-administered with duloxetine (60 mg twice daily). Serotonin release by platelets plays an important role in hemostasis. Epidemiological studies of the case-control and cohort design that have demonstrated an association between use of psychotropic drugs that interfere with serotonin reuptake and the occurrence of upper gastrointestinal bleeding have also shown that concurrent use of an NSAID or aspirin may potentiate this risk of bleeding. Altered anticoagulant effects, including increased bleeding, have been reported when SSRIs or SNRIs are co-administered with warfarin. Concomitant administration of warfarin (2-9 mg once daily) under steady state conditions with duloxetine 60 or 120 mg once daily for up to 14 days in healthy subjects (n=15) did not significantly change INR from baseline (mean INR changes ranged from 0.05 to +0.07). The total warfarin (protein bound plus free drug) pharmacokinetics (AUCt,ss, Cmax,ss or tmax,ss) for both R- and S-warfarin were not altered by duloxetine. Because of the potential effect of duloxetine on platelets, patients receiving warfarin therapy should be carefully monitored when duloxetine is initiated or discontinued. Concomitant administration of duloxetine 40 mg twice daily with fluvoxamine 100 mg, a potent CYP1A2 inhibitor, to CYP2D6 poor metabolizer subjects (n=14) resulted in a 6-fold increase in duloxetine AUC and Cmax. Concomitant use of duloxetine (40 mg once daily) with paroxetine (20 mg once daily) increased the concentration of duloxetine AUC by about 60%, and greater degrees of inhibition are expected with higher doses of paroxetine. Similar effects would be expected with other potent CYP2D6 inhibitors (e.g., fluoxetine, quinidine). When duloxetine 60 mg was co-administered with fluvoxamine 100 mg, a potent CYP1A2 inhibitor, to male subjects (n=14) duloxetine AUC was increased approximately 6-fold, the Cmax was increased about 2.5-fold, and duloxetine t1/2 was increased approximately 3-fold. Other drugs that inhibit CYP1A2 metabolism include cimetidine and quinolone antimicrobials such as ciprofloxacin and enoxacin. |
| 参考文献 |
[1]. Clin Pharmacokinet . 2011 May;50(5):281-94. [2]. Duloxetine-Induced Neural Cell Death and Promoted Neurite Outgrowth in N2a Cells. Neurotox Res. 2020 Dec;38(4):859-870.[3]. Duloxetine Protects against Oxaliplatin-Induced Neuropathic Pain and Spinal Neuron Hyperexcitability in Rodents. Int J Mol Sci . 2017 Dec 5;18(12):2626. |
| 其他信息 |
Therapeutic Uses
Adrenergic Uptake Inhibitors; Analgesics; Antidepressive Agents; Dopamine Uptake Inhibitors; Serotonin Uptake Inhibitors Duloxetine hydrochloride is used for the acute and maintenance treatment of major depressive disorder in adults. Duloxetine has been used for the management of moderate to severe stress urinary incontinence (SUI) in women. Duloxetine hydrochloride is used for the management of fibromyalgia in adults. For more Therapeutic Uses (Complete) data for DULOXETINE (6 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ WARNING: SUICIDAL THOUGHTS AND BEHAVIORS: Antidepressants increased the risk of suicidal thoughts and behavior in children, adolescents, and young adults in short-term studies. These studies did not show an increase in the risk of suicidal thoughts and behavior with antidepressant use in patients over age 24; there was a reduction in risk with antidepressant use in patients aged 65 and older. In patients of all ages who are started on antidepressant therapy, monitor closely for worsening, and for emergence of suicidal thoughts and behaviors. Advise families and caregivers of the need for close observation and communication with the prescriber. Pregnancy Category C. Some neonates exposed to selective serotonin- and norepinephrine-reuptake inhibitors (SNRIs) or selective serotonin-reuptake inhibitors late in the third trimester of pregnancy have developed complications that have sometimes been severe and required prolonged hospitalization, respiratory support, enteral nutrition, and other forms of supportive care in special-care nurseries. Such complications can arise immediately upon delivery and usually last several days or up to 2-4 weeks. Clinical findings reported to date in the neonates have included respiratory distress, cyanosis, apnea, seizures, temperature instability or fever, feeding difficulty, dehydration, excessive weight loss, vomiting, hypoglycemia, hypotonia, hyperreflexia, tremor, jitteriness, irritability, lethargy, reduced or lack of reaction to pain stimuli, and constant crying. These clinical features appear to be consistent with either a direct toxic effect of the SNRI or selective serotonin-reuptake inhibitor or, possibly, a drug withdrawal syndrome. It should be noted that, in some cases, the clinical picture was consistent with serotonin syndrome (see Drug Interactions: Drugs Associated with Serotonin Syndrome, in Fluoxetine Hydrochloride 28:16.04.20). When treating a pregnant woman with duloxetine during the third trimester of pregnancy, the clinician should carefully consider the potential risks and benefits of such therapy. Consideration may be given to cautiously tapering duloxetine therapy in the third trimester prior to delivery if the drug is administered during pregnancy. Potentially life-threatening serotonin syndrome reported with selective serotonin- and norepinephrine-reuptake inhibitors (SNRIs), including duloxetine, or selective serotonin-reuptake inhibitors (SSRIs), particularly with concurrent administration of other serotonergic drugs (e.g., serotonin [5-hydroxytryptamine; 5-HT] type 1 receptor agonists ["triptans"]) or drugs that impair serotonin metabolism (e.g., monoamine oxidase [MAO] inhibitors). Symptoms of serotonin syndrome may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination), and/or GI symptoms (e.g., nausea, vomiting, diarrhea). Concurrent therapy with MAO inhibitors used for treatment of depression is contraindicated. (See Drug Interactions: Monoamine Oxidase Inhibitors.) If concurrent therapy with duloxetine and a 5-HT1 receptor agonist is clinically warranted, the patient should be observed carefully, particularly during initiation of therapy, when dosage is increased, or when another serotonergic agent is initiated. Concomitant use of duloxetine and serotonin precursors (e.g., tryptophan) is not recommended. Hepatic failure, sometimes fatal, has been reported in duloxetine-treated patients.The cases presented as hepatitis accompanied by abdominal pain, hepatomegaly, and markedly elevated serum transaminase concentrations (more than 20 times the upper limit of normal) with or without jaundice, reflecting a mixed or hepatocellular pattern of hepatic injury. Duloxetine should be discontinued in any patient who develops jaundice or other evidence of clinically important hepatic dysfunction; therapy should not be resumed unless another cause for the hepatic dysfunction can be established. For more Drug Warnings (Complete) data for DULOXETINE (18 total), please visit the HSDB record page. Pharmacodynamics Duloxetine, through increasing serotonin and norepinephrine concentrations in Onuf's nucleus, enhances glutamatergic activation of the pudendal motor nerve which innervates the external urethral sphinter. This enhanced signaling allows for stronger contraction. Increased contraction of this sphincter increases the pressure needed to produce an incontinence episode in stress urinary incontinence. Duloxetine has been shown to improve Patient Global Impression of Improvement and Incontinence Quality of Life scores. It has also been shown to reduce the median incontinence episode frequency at doses of 40 and 80 mg. Action at the dorsal horn of the spinal cord allows duloxetine to strengthen the the serotonergic and adrenergic pathways involved in descending inhibition of pain. This results in an increased threshold of activation necessary to transmit painful stimuli to the brain and effective relief of pain, particularly in neuropathic pain. Pain relief has been noted in a variety of painful conditions including diabetic peripheral neuropathy, fibromyalgia, and osteoarthritis using a range of pain assessment surveys. While duloxetine has been shown to be effective in both animal models of mood disorders and in clinical trials for the treatment of these disorders in humans, the broad scope of its pharmacodynamic effects on mood regulation in the brain has yet to be explained. Increased blood pressure is a common side effect with duloxetine due to vasoconstriction mediated by the intended increase in norepinephrine signaling. |
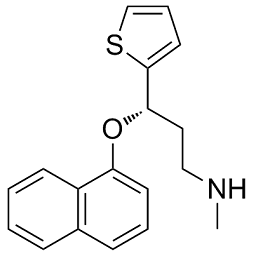
| 分子式 |
C18H19NOS
|
|---|---|
| 分子量 |
297.416
|
| 精确质量 |
297.12
|
| CAS号 |
116539-59-4
|
| 相关CAS号 |
Duloxetine hydrochloride; 136434-34-9; Duloxetine-d7; 919514-01-5; (±)-Duloxetine hydrochloride; 947316-47-4; Duloxetine metabolite Para-Naphthol Duloxetine; 949095-98-1
|
| PubChem CID |
60835
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
466.2±40.0 °C at 760 mmHg
|
| 闪点 |
235.7±27.3 °C
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
| 折射率 |
1.628
|
| LogP |
3.73
|
| tPSA |
49.5
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
21
|
| 分子复杂度/Complexity |
312
|
| 定义原子立体中心数目 |
1
|
| SMILES |
CNCC[C@H](OC1=CC=CC2=C1C=CC=C2)C3=CC=CS3
|
| InChi Key |
ZEUITGRIYCTCEM-KRWDZBQOSA-N
|
| InChi Code |
InChI=1S/C18H19NOS/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16/h2-10,13,17,19H,11-12H2,1H3/t17-/m0/s1
|
| 化学名 |
(3S)-N-methyl-3-naphthalen-1-yloxy-3-thiophen-2-ylpropan-1-amine
|
| 别名 |
LY248686; LY-227942; LY-248686; LY 227942; (S)-Duloxetine; (S)-Duloxetine; Yentreve; Cymbalta; (S)-N-Methyl-3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-1-amine; LY 248686; HSDB 7368; Duloxetine
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.3622 mL | 16.8112 mL | 33.6225 mL | |
| 5 mM | 0.6724 mL | 3.3622 mL | 6.7245 mL | |
| 10 mM | 0.3362 mL | 1.6811 mL | 3.3622 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Comparing Effectiveness of Duloxetine and Desipramine in Patients With Chronic Pain: A Pragmatic Trial Using Point of Care Randomization
CTID: NCT03548454
Phase: Phase 4 Status: Recruiting
Date: 2024-11-22