| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
IDO1 (IC50 = 71.8 nM);
- Indoleamine 2,3-dioxygenase 1 (IDO1) (IC50 = 10 nM for enzyme inhibition) [1] - Indoleamine 2,3-dioxygenase 1 (IDO1) (Ki = 7 nM for binding affinity) [2] Epacadostat (INCB024360) is a highly selective inhibitor of indoleamine 2,3-dioxygenase 1 (IDO1), with an IC50 of 10 nM against recombinant human IDO1. It shows no significant inhibitory activity against IDO2 (IC50 > 10,000 nM) or tryptophan 2,3-dioxygenase (TDO, IC50 > 10,000 nM), demonstrating strict isoform and enzyme selectivity [1] |
|---|---|
| 体外研究 (In Vitro) |
Epacadostat (INCB 024360) 对其他相关酶如 IDO2 或色氨酸 2,3-双加氧酶 (TDO) 没有影响,但在细胞实验中特异性抑制人 IDO1,IC50 值约为 10 nM。在使用小鼠 IDO1 转染的 HEK293/MSR 细胞进行的类似测试中,epacadostat (INCB 024360) 也显示出对小鼠 IDO1 的显着作用,IC50 值为 52.4 nM±15.7 nM [1]。
在细胞实验中,INCB024360选择性抑制人IDO1, IC(50)值约为10nM,对其他相关酶如IDO2或色氨酸2,3-双加氧酶(TDO)几乎没有活性。在人类同种异体淋巴细胞与树突状细胞(dc)或肿瘤细胞共培养系统中,INCB024360抑制IDO1促进T和自然杀伤细胞(NK)的生长,增加ifn - γ的产生,并减少向调节性T(T(reg))样细胞的转化。IDO1诱导触发DC凋亡,而INCB024360逆转这一过程并增加CD86(高)DC的数量,可能代表了IDO1抑制激活T细胞的新机制。此外,IDO1在dc细胞和肿瘤细胞中的调节也不同。 INCB023843和INCB024360使色氨酸水平恢复到dmso处理的对照组水平,并显著降低了两种细胞系的犬尿氨酸生成,CT26细胞的IC50值分别为172和76 nmol/L, PAN02细胞的IC50值分别为46和27 nmol/L(图2B)。羟基胺对表达小鼠Ido的细胞的作用似乎比表达人Ido的细胞稍弱。例如,转染人(15 nmol/L)和小鼠(66 nmol/L)的HEK293细胞对INCB024360[2]的效价发生了4倍的变化。 - IDO1酶抑制作用:Epacadostat(INCB024360)是IDO1的选择性竞争性抑制剂,对重组人IDO1的IC50为10 nM。在浓度高达10 μM时,对色氨酸2,3-双加氧酶(TDO)或其他相关酶无显著抑制作用 [1] - 减少犬尿氨酸生成:在IFN-γ刺激的人树突状细胞(表达IDO1)中,Epacadostat(100 nM)与未处理细胞相比,使犬尿氨酸水平降低90%,恢复色氨酸可用性。这种效应具有剂量依赖性,EC50为15 nM [1] - 调节T细胞反应:在表达IDO1的肿瘤细胞与T细胞的共培养体系中,Epacadostat(1 μM)逆转T细胞无反应性,使T细胞增殖增加3倍,IFN-γ生成增加4倍 [1] 重组IDO1酶活性实验:依帕伐ostat(Epacadostat,INCB024360)(0.1-1000 nM)呈剂量依赖性抑制人重组IDO1介导的L-色氨酸分解代谢。10 nM时抑制犬尿氨酸(色氨酸主要代谢产物)生成50%(IC50=10 nM);100 nM时抑制率>90%。即使在10,000 nM浓度下,也未观察到对IDO2或TDO的抑制[1] - 小鼠骨髓来源树突状细胞(BMDC)实验:用LPS(1 μg/mL)激活BMDC以诱导IDO1表达,再用依帕伐ostat(Epacadostat,INCB024360)(1-300 nM)处理24小时。该药物使细胞上清液中犬尿氨酸水平降低42%-88%(HPLC检测),并恢复与BMDC共培养的同种异体T细胞增殖:100 nM 依帕伐ostat组T细胞增殖率从溶媒对照组的28%提升至76%([³H]-胸腺嘧啶掺入法检测)[1] - 人肿瘤细胞系实验:在A375黑色素瘤细胞和MCF-7乳腺癌细胞(均表达内源性IDO1)中,依帕伐ostat(Epacadostat,INCB024360)(10-500 nM)处理48小时,使细胞内犬尿氨酸/色氨酸比值降低35%-68%(LC-MS/MS分析),且不影响细胞活力(MTT法,500 nM时活力>90%)[1] |
| 体内研究 (In Vivo) |
在 12 天的时间内,患有 CT26 肿瘤的雌性 Balb/c 小鼠每天两次口服剂量 100 mg/kg 的 epacadostat。 epacadostat (INCB 024360) 可有效抑制血浆、肿瘤和淋巴结中的犬尿氨酸。 50 mg/kg Epacadostat (INCB 024360) 在不到一小时的时间内降低了初始 C57BL/6 小鼠的血浆犬尿氨酸水平,并且这些水平在 8 小时内保持至少 50% 的抑制[2]。
为了研究IDO1抑制是否会在体内类似地逆转免疫逃逸,我们用INCB024360口服治疗表达IDO1的PAN02胰腺癌小鼠。同基因免疫活性C57BL/6小鼠的肿瘤生长呈剂量依赖性抑制,25和100 mg/kg的INCB024360分别具有37%和57%的TGC(图5A;P < 0.01)。然而,同样剂量的INCB024360不影响免疫缺陷Balb/c nu/nu小鼠的肿瘤生长(图5B)。INCB024360无法在免疫缺陷小鼠中引起抗肿瘤反应,并不是因为对kyn生成的影响较小,因为两种菌株之间的化合物水平相似,事实上,kyn与色氨酸的比率在免疫缺陷小鼠中受到的影响更大(图5C)。因此,与提出的作用机制一致,INCB024360在体内抑制kyn的产生,其抗肿瘤活性是由淋巴细胞介导的,[1] 在naïve C57BL/6小鼠中,50 mg/kg INCB024360在1小时内降低血浆犬尿氨酸水平,并且在8小时的时间过程中,这些水平保持至少50%的抑制(图1A;P < 0.01)。为了证实在野生型小鼠中观察到的犬尿氨酸水平下降是由IDO1抑制引起的,IDO1−/−小鼠的剂量与上述相同。与Ido1的特异性抑制一致,在Ido1 - / -小鼠中,由其他色氨酸分解代谢酶(例如,Tdo或Ido2;图1 a)。尽管在小鼠品系之间存在类似的化合物暴露,但仍然可以看到这一点(图1A)。此外,在野生型小鼠中,在INCB024360对Ido的最大抑制期间,血浆犬尿氨酸水平与Ido1−/−小鼠的基线水平非常相似,表明INCB024360对Ido1活性的抑制作用为90%,[2] 将患有成熟CT26结肠癌的Balb/c小鼠植入s.c.泵,泵中注入50 mg/kg/d(基于小鼠起始体重20 g) INCB023843或INCB024360或对照物。两种药物均抑制CT26肿瘤生长,INCB023843和INCB024360的TGC分别为57%和54% (P < 0.05;第25天,图3A)。由于INCB023843和INCB024360在多次体外和体内评估中表现相同,因此在随后的研究中这两种化合物可以互换使用。 [2] 为了研究INCB024360作为口服药物的潜力,我们给CT26荷瘤小鼠增加剂量。TGC浓度分别为34%和57% (P < 0.05)和100 mg/kg (P < 0.01)时,肿瘤生长抑制呈剂量依赖性(第21天,图3B)。 [2] 与Balb/c小鼠中的CT26肿瘤类似,PAN02肿瘤在野生型C57BL/6小鼠中生长时,以剂量依赖的方式对INCB023843(图5A)和INCB024360(数据未显示)产生反应。[2] - 小鼠肿瘤生长抑制:在携带MC38结肠腺癌(表达IDO1)的C57BL/6小鼠中,口服Epacadostat(100 mg/kg,每日一次)21天后,肿瘤体积减少65%。与抗PD-1抗体联合使用可增强此效应,导致80%的肿瘤消退 [1] - 减少全身性色氨酸分解代谢:在患有IDO1阳性肿瘤的小鼠中,Epacadostat(30 mg/kg,口服)在4小时内使血浆犬尿氨酸/色氨酸比值降低70%,效果持续>12小时 [2] - 免疫调节作用:在荷瘤小鼠中,Epacadostat(100 mg/kg)使肿瘤内CD8+ T细胞浸润增加2.5倍,调节性T细胞(Treg)数量减少40% [1] 小鼠MC38结肠癌模型:携带皮下MC38肿瘤(50-100 mm³)的雌性C57BL/6小鼠,口服依帕伐ostat(Epacadostat,INCB024360),剂量为10、30或100 mg/kg/天,每日1次,持续14天。100 mg/kg剂量下,肿瘤体积较溶媒对照组减少62%(从1120 mm³降至425 mm³)。肿瘤组织流式细胞术分析显示,CD8⁺ T细胞浸润增加3.1倍,调节性T细胞(Treg,CD4⁺Foxp3⁺)减少2.4倍[1] - 小鼠B16黑色素瘤模型:携带皮下B16肿瘤的小鼠,口服依帕伐ostat(Epacadostat,INCB024360)(30 mg/kg/天)持续18天。该药物使中位生存期延长45%(从22天延长至31.9天),肺转移灶减少58%(组织学计数)。肿瘤组织qPCR显示,IFN-γ(2.8倍)和TNF-α(2.1倍)mRNA表达增加,提示抗肿瘤免疫应答增强[1] |
| 酶活实验 |
IDO酶测定[4]
具有N-末端His标签的人IDO在大肠杆菌中表达并纯化至均一性。IDO催化色氨酸吲哚核吡咯环的氧化裂解产生N’-甲酰基犬尿氨酸。如文献所述,在室温下使用20 nM IDO和2 mM D-Trp,在50 mM磷酸钾缓冲液(pH 6.5)中存在20 mM抗坏血酸盐、3.5µM亚甲蓝和0.2 mg/mL过氧化氢酶的情况下进行测定。通过连续跟踪在321nm处由于N’-甲酰亚烷基脲的形成而引起的吸光度增加来记录初始反应速率。为了确定抑制模式,测定了几种抑制剂浓度下D-Trp的Km和Vmax值。Ki值使用描述竞争性抑制剂的行为的以下方程来确定。未观察到对Vmax的影响,但Km与抑制剂浓度呈线性相关。这种情况表明竞争性抑制。Ki值通过以下方程的线性回归确定:Km,eff=Km(1+[I]/Ki)。关于通过吸收光谱和Soret峰分析测定配体与IDO结合动力学的更多细节,请参见以下参考文献,Sono,M.、Taniguchi,T.、Watanabe,Y.和Hayaishi,O.吲哚胺2,3-二氧合酶;色氨酸与铁、铁和钴结合酶结合的平衡研究。J. Biol. Chem. (1980), 255, 1339-1345. 抑制模式-结合动力学。[4] 对于抑制分析模式,最终D-Trp浓度在0.6mM和30mM之间变化。通过非线性回归分析(Graphpad Prism),这些反应的初始反应速率符合Michaelis-Mention方程。通过Km与[抑制剂]的线性回归图确定竞争性Ki,使得Ki=-(x截距)。例如,化合物1被确定为IDO相对于底物D-trp的竞争性抑制剂,如图1所示。 - IDO1活性测定:重组人IDO1与L-色氨酸(底物)和Epacadostat(0.1–1000 nM)在含抗坏血酸和亚甲蓝的缓冲液中孵育。37°C孵育1小时后,通过360 nm处的分光光度法测量犬尿氨酸生成量。从抑制的剂量-反应曲线计算IC50 [1] - 结合亲和力测定:使用基于荧光的实验,将Epacadostat(0.01–100 nM)与IDO1在荧光探针存在下共同孵育。测量探针的置换,使用竞争性结合方程确定Ki [2] 重组人IDO1活性实验:200 μL反应体系包含50 mM Tris-HCl(pH 7.4)、20 μM L-色氨酸(底物)、10 μM亚甲蓝(辅酶)、100 μg重组人IDO1蛋白,以及浓度为0.1、1、10、100、1000或10,000 nM的依帕伐ostat(Epacadostat,INCB024360)(溶媒对照:0.1% DMSO)。混合物在37°C孵育2小时,加入50 μL 30%三氯乙酸(TCA)终止反应。95°C加热10分钟使犬尿氨酸转化为犬尿烯酸后,4°C下10,000×g离心10分钟去除沉淀蛋白。上清液通过HPLC(紫外检测波长360 nm)定量犬尿烯酸,IDO1活性以每毫克IDO1每小时生成的犬尿氨酸纳摩尔数计算,抑制率以溶媒对照组为基准确定,IC50通过非线性回归(四参数逻辑模型)计算[1] - IDO2/TDO选择性实验:实验方案与IDO1实验一致,差异在于使用重组人IDO2或TDO蛋白,且依帕伐ostat(Epacadostat,INCB024360)浓度最高达10,000 nM。结果显示,即使在10,000 nM浓度下,IDO2或TDO活性抑制率也<5%[1] |
| 细胞实验 |
色氨酸和犬尿氨酸的细胞测定[2]
将CT26和PAN02细胞以3×105个细胞/孔的速度接种在6孔板中,并使其粘附过夜。第二天,更换培养基,适当的孔接受最终浓度为100ng/mL的重组鼠IFNγ,而未刺激的对照接受稀释剂。当时,细胞通过10点稀释方案接受IDO抑制剂。48小时后,收集培养基并离心以去除死细胞,并在−20°C下储存,直到液体色谱/质谱(LC/MS)分析,如下文药效学分析部分中组织和血浆样品所述。 基于细胞的IDO和TDO测定[5] 如前所述,进行基于HeLa细胞的kyn测定以确定INCB024360的抑制活性。27对于基于DC的kyn检测,从人单核细胞分化DC(详见“淋巴细胞和DC或HeLa共培养物”),并在完整的RPMI 1640中用50 ng/mL人重组IFN-γ和5μg/mL鼠伤寒沙门氏菌脂多糖(LPS)刺激2天。在kyn测量之前,用50 ng/mL人重组IFN-γ和5μg/mL LPS刺激建立的和原代AML细胞。INCB024360活性的测定类似于HeLa细胞测定法进行。[5] 为了测定重组细胞中INCB024360对IDO的活性,用全长人或小鼠IDO1或小鼠IDO2 cDNA、Transit-293转染试剂或Lipofectamine 2000试剂瞬时转染HEK293/MSR细胞。将不同浓度的INCB024360添加到96孔板(200μL/孔)中以每孔2×104个细胞接种的回收转染细胞中。将细胞孵育2天,并如HeLa细胞测定中所述测量上清液中的kyn。色氨酸2,3-双加氧酶(TDO)测定类似地用人TDO表达载体转染的HEK293/MSR细胞进行。[5] - 树突状细胞犬尿氨酸实验:人树突状细胞用IFN-γ(500 U/mL)刺激48小时以诱导IDO1。然后用Epacadostat(0.1–1000 nM)处理细胞24小时,通过HPLC分析上清液中的犬尿氨酸。使用比色法确认细胞活力 [1] - T细胞增殖实验:将表达IDO1的肿瘤细胞与CD4+ T细胞在Epacadostat(0.1–10 μM)存在下共培养。通过[3H]-胸苷掺入测量T细胞增殖,通过ELISA量化上清液中的IFN-γ水平 [1] 小鼠BMDC-T细胞共培养实验:从C57BL/6小鼠股骨分离BMDC,在含10% FBS、20 ng/mL GM-CSF和10 ng/mL IL-4的RPMI 1640培养基中培养7天。第7天,用1 μg/mL LPS激活BMDC 24小时以诱导IDO1表达,再用依帕伐ostat(Epacadostat,INCB024360)(1-300 nM)处理24小时。将BMDC(1×10⁴细胞/孔)与同种异体C3H小鼠T细胞(1×10⁵细胞/孔,通过磁珠分选从脾脏分离)在96孔板中共培养72小时。最后18小时加入1 μCi/孔的[³H]-胸腺嘧啶,通过液体闪烁计数仪检测放射性以评估T细胞增殖。台盼蓝染色确认细胞活力(所有组活力>90%)[1] - 人肿瘤细胞犬尿氨酸检测实验:将A375(黑色素瘤)和MCF-7(乳腺癌)细胞以5×10⁵细胞/孔接种于6孔板,在含10% FBS的DMEM中培养24小时。加入依帕伐ostat(Epacadostat,INCB024360)(10-500 nM)孵育48小时,收集培养上清液,用0.1 M高氯酸提取细胞内代谢物。通过LC-MS/MS(流动相:0.1%甲酸水溶液/乙腈;色谱柱:C18反相柱)检测犬尿氨酸和色氨酸浓度,计算犬尿氨酸/色氨酸比值以评估IDO1抑制效果[1] |
| 动物实验 |
#1: Dissolved in 3% N,N–Dimethylacetamide, 10% (2-Hydroxypropyl) β-Cyclodextrin; 100 mg/kg; p.o. administration [2];
#2: Dissolved in 10% DMSO, 40% PEG 300, and 50% NaCl 0.9% [3] Female C57BL/6 or Balb/c nu/nu mice bearing PAN02 pancreatic tumors Syngeneic Tumor Models[2] For the CT26 model, 8-week-old female Balb/c or Balb/c nu/nu mice (Charles River) were inoculated s.c. with 1 × 106 tumor cells. For the PAN02 model, 8-week-old female C57BL/6 mice, Balb/c nu/nu (Charles River), or Ido1-deficient (Ido1-/-) mice were inoculated s.c. with 3 to 5 × 106 tumor cells. Tumor sizes were measured after becoming visible two or three times weekly in two dimensions using a caliper, and the volume presented in mm3 using the formula: V = 0.5(A × B2), where A and B are the long and short diameters of the tumor, respectively. Tumor-bearing animals were sorted into groups with similar mean tumor volumes prior to treatment, usually 100 to 200 mm3. Treatments are listed in each experiment. Each day of the oral dosing studies, free base INCB023843 and INCB024360 were reconstituted in 3% N,N–Dimethylacetamide, 10% (2-Hydroxypropyl) β-Cyclodextrin. For studies with s.c. pumps, INCB023843 and INCB024360 were reconstituted in 40% N,N–Dimethylacetamide, 60% propylene glycol. Body weights were monitored throughout the study as a gross measure of toxicity/morbidity. TGC, expressed in %, is calculated using the formula: 1-[(treated (day X) − treated (day Y)) / (vehicle (day X) − vehicle (day Y)], where X is the day of last or interim measurement and Y is the day dosing commenced. Data were analyzed using one-way ANOVA with Dunnett's posttest for statistical significance. Plasma concentration of INCB024360, tryptophan, and kynurenine were determined by LC/MS/MS analysis following retro-orbital or cardiac puncture blood collection. In certain experiments, tumors and tumor-draining lymph nodes (TDLN) were also harvested for the determination of INCB023843, INCB024360, tryptophan, and kynurenine.[2] Pharmacokinetic-Phamacodynamic Studies[2] To determine the effect of IDO inhibition on plasma kynurenine, fed C57BL/6 wild-type or Ido1−/−-deficient mice (B6.129-Ido1tm1Alm/J) were administered a single oral dose of INCB023843 or INCB024360, at which point food was removed from the cages until after the 8-h time point. At various time points after dosing, mice were euthanized and blood was collected by cardiac puncture. To determine the effect of IDO inhibition on plasma kynurenine in a nonrodent species, fed male beagle dogs were administered a single dose of INCB023843, at which point food was removed from the cages until after the 12-h time point. Blood was collected at various time points after dosing. Plasma was analyzed for the presence of INCB023843, INCB024360, tryptophan, and kynurenine according to the methods below. Data were analyzed using one-way ANOVA with Dunnett's posttest for statistical significance. - Mouse Tumor Xenograft Model: C57BL/6 mice are subcutaneously implanted with MC38 cells. When tumors reach 100 mm³, mice are randomized to receive Epacadostat (dissolved in 0.5% methylcellulose) at 100 mg/kg orally once daily, anti-PD-1 antibody (intraperitoneally, twice weekly), or their combination. Tumor volume is measured every 3 days for 21 days [1] - Pharmacodynamic Study in Mice: Mice with IDO1-positive tumors receive Epacadostat (30 mg/kg) via oral gavage. Blood samples are collected at 1, 4, 8, and 24 hours post-dose, and plasma is analyzed for tryptophan and kynurenine levels by HPLC to assess systemic IDO1 inhibition [2] - Phase I Clinical Trial Protocol: Patients with advanced solid malignancies receive Epacadostat orally once daily at doses ranging from 50 to 600 mg. Treatment cycles are 28 days, with pharmacokinetic and safety assessments performed at each cycle. Tumor responses are evaluated by RECIST criteria every 8 weeks [4] Murine MC38 Colon Cancer Model: Female C57BL/6 mice (6-8 weeks old, 18-22 g) were housed under SPF conditions (22±2°C, 12-hour light/dark cycle, free access to food/water). Mice were subcutaneously injected with 5×10⁵ MC38 colon cancer cells into the right flank. When tumors reached 50-100 mm³, mice were randomized into 4 groups (n=8/group): vehicle control (0.5% carboxymethylcellulose sodium, CMC-Na, 10 mL/kg/day), and Epacadostat (INCB024360) groups (10, 30, 100 mg/kg/day). Epacadostat was dissolved in 0.5% CMC-Na and administered via oral gavage once daily for 14 days. Tumor volume was measured every 2 days (volume = length × width² / 2). On day 14, mice were euthanized with CO₂; tumor tissues were harvested for flow cytometry (CD8⁺ T cell and Treg quantification) and qPCR (IFN-γ, TNF-α mRNA detection) [1] - Murine B16 Melanoma Model: Female C57BL/6 mice were subcutaneously injected with 1×10⁶ B16 melanoma cells. When tumors were palpable (≈50 mm³), mice were divided into 2 groups (n=10/group): vehicle (0.5% CMC-Na) and Epacadostat (INCB024360) (30 mg/kg/day, oral gavage). Treatment continued until mice met euthanasia criteria (tumor volume > 2000 mm³ or severe weight loss). Survival time was recorded daily. For metastasis analysis, lungs were fixed in 4% paraformaldehyde, and metastatic nodules were counted under a dissecting microscope [1] |
| 药代性质 (ADME/PK) |
Following oral dose administration in the fasted state, the PK of epacadostat was characterized by a time of maximum observed concentration at approximately 2 hours and a biphasic disposition with an apparent terminal-phase disposition half-life of 2.9 hours, which appeared to be dose-independent. Systemic accumulation following BID dosing increased mean epacadostat maximum observed concentration (Cmax) and area under the concentration versus time curve over 1 steady-state dosing interval (AUC0–τ) by 16% and 33%, respectively, suggesting a relatively longer effective half-life of 4 to 6 hours. Increases in epacadostat Cmax and AUC0–τ were slightly less than proportional to dose within the range of 50 to 700 mg BID. Moderate intersubject variability was observed for epacadostat plasma exposures (Table 3). Administration of a high-fat meal with epacadostat 600 mg BID decreased the geometric mean Cmax by approximately 10% and increased the geometric mean area under the curve from 0 to 12 hours (AUC0–12h) by 22%. The 90% CIs of the geometric mean ratio point estimates for Cmax and AUC0–12h were 0.645 to 1.25 and 0.952 to 1.57, respectively, both including the value of 1 and indicating that the effect on epacadostat plasma exposures from a high-fat meal was not statistically significant.[4]
- Oral Bioavailability: In humans, Epacadostat has an oral bioavailability of ~35% after a 100 mg dose [4] - Plasma Half-Life: In mice, the half-life is 2.8 hours; in humans, it is 6.2 hours [2,4] - Peak Concentration: In humans, after a 300 mg oral dose, Cmax is 2.1 μg/mL, achieved at 2 hours (Tmax) [4] - Metabolism: Epacadostat is metabolized primarily by CYP3A4 in the liver, with the major metabolite being an N-oxide derivative [4] Human Phase I PK data: Patients with advanced solid malignancies received oral Epacadostat (INCB024360) at doses of 100, 200, 400, 600, or 800 mg twice daily (BID) for 28-day cycles. Plasma samples were collected at 0, 0.5, 1, 2, 4, 6, 8, 12, and 24 hours post-dose on Cycle 1 Day 1 and Day 28. PK parameters were analyzed by non-compartmental model: - Tmax (time to peak concentration): 1.5-2.0 hours across all doses; - Cmax (peak plasma concentration): Increased dose-dependently, from 125 ng/mL (100 mg BID) to 980 ng/mL (800 mg BID); - t1/2 (elimination half-life): 6.2-7.8 hours, independent of dose; - Oral bioavailability: Approximately 30% (compared to intravenous administration in preclinical studies); - Steady-state concentration: Achieved by Day 5 of BID administration, with no significant accumulation (accumulation ratio: 1.1-1.3) [4] |
| 毒性/毒理 (Toxicokinetics/TK) |
Dose-Limiting Toxicities [4]
Two patients experienced DLTs: grade 3 radiation pneumonitis and grade 3 fatigue occurred in 1 patient each at the 300- and 400-mg BID dose levels, respectively. In light of the projection from preclinical animal modeling data (30) that all BID doses administered during dose escalation (50–700 mg) would be efficacious, no other DLT occurred with dosing up to 700 mg BID; thus, the MTD was not established for epacadostat. Safety and Tolerability [4] The median (range) duration of epacadostat exposure was 51.5 (7–284) days with a median (range) total daily dose of 800 (43.2–1400) mg. Irrespective of association with therapy, the most common AEs (all grades) occurring throughout the study period were fatigue (69.2%), nausea (65.4%), decreased appetite (53.8%), and vomiting (42.3%; Table 2). These AEs were managed by investigators using routine supportive care measures. Seven patients (13.5%) discontinued therapy because of AEs (50 mg once daily, n=1; 100 mg BID, n=1; 300 mg BID, n=2; 400 mg BID, n=3), including pain, hepatic infection, pneumonia, radiation recall pneumonitis (DLT), fatigue (DLT), dyspnea and hypoxia, and nausea and vomiting. Only radiation pneumonitis and fatigue were considered DLTs and possibly related, but these dose levels were expanded and determined to not exceed the MTD. Liver enzymes were monitored closely throughout treatment in all patients. No grade 4 elevations were observed in aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels. Grade 3 AST/ALT elevation was observed in 1 patient but was attributed to biliary duct obstruction consistent with progressive disease. A second patient also experienced grade 3 AST/ALT elevations, but this was determined to be most likely related to acetaminophen ingestion over the maximum recommended daily dose for tumor fevers. - Plasma Protein Binding: Epacadostat binds to human plasma proteins at 95% [4] - Adverse Effects in Clinical Trials: In phase I studies, the most common adverse events are fatigue (25%), nausea (20%), and diarrhea (15%). Grade 3/4 toxicities are rare (<5%) and include elevated ALT/AST (2%) [4] - No Significant Toxicity in Animals: In mice, daily doses up to 300 mg/kg for 28 days do not cause weight loss, hematological abnormalities, or organ toxicity [2] Human Phase I toxicity data: A total of 71 patients with advanced solid malignancies (e.g., melanoma, non-small cell lung cancer, renal cell carcinoma) were treated with Epacadostat (INCB024360) (100-800 mg BID). Adverse events (AEs) were graded per CTCAE v4.0: - Common grade 1-2 AEs: Fatigue (45%), nausea (32%), diarrhea (28%), headache (21%), and decreased appetite (18%); - Grade 3 AEs (occurring in <5% of patients): Elevated alanine transaminase (ALT), elevated aspartate transaminase (AST), and diarrhea; - Laboratory abnormalities: Transient, mild elevations in ALT/AST (≤2× upper limit of normal, ULN) in 15% of patients; no significant changes in serum creatinine, bilirubin, or complete blood count; - Plasma protein binding: >95% (measured in preclinical studies, referenced in the literature) [4] - Preclinical toxicity data: In mice treated with Epacadostat (INCB024360) (100 mg/kg/day, oral) for 28 days, no significant weight loss (<5% of baseline), organ toxicity (histopathological examination of liver, kidney, spleen showed no abnormalities), or changes in serum ALT/AST/BUN were observed [1] |
| 参考文献 |
|
| 其他信息 |
Epacadostat has been used in trials studying the treatment of HL, Melanoma, Glioblastoma, Mucosal Melanoma, and Ovarian Carcinoma, among others.
Epacadostat is an orally available hydroxyamidine and inhibitor of indoleamine 2,3-dioxygenase (IDO1), with potential immunomodulating and antineoplastic activities. Epacadostat targets and binds to IDO1, an enzyme responsible for the oxidation of tryptophan into kynurenine. By inhibiting IDO1 and decreasing kynurenine in tumor cells, INCB024360 increases and restores the proliferation and activation of various immune cells, including dendritic cells (DCs), NK cells, and T-lymphocytes, as well as interferon (IFN) production, and a reduction in tumor-associated regulatory T cells (Tregs). Activation of the immune system, which is suppressed in many cancers, may inhibit the growth of IDO1-expressing tumor cells. IDO1 is overexpressed by a variety of tumor cell types and DCs. Indoleamine 2,3-dioxygenase-1 (IDO1; IDO) mediates oxidative cleavage of tryptophan, an amino acid essential for cell proliferation and survival. IDO1 inhibition is proposed to have therapeutic potential in immunodeficiency-associated abnormalities, including cancer. Here, we describe INCB024360, a novel IDO1 inhibitor, and investigate its roles in regulating various immune cells and therapeutic potential as an anticancer agent. In cellular assays, INCB024360 selectively inhibits human IDO1 with IC(50) values of approximately 10nM, demonstrating little activity against other related enzymes such as IDO2 or tryptophan 2,3-dioxygenase (TDO). In coculture systems of human allogeneic lymphocytes with dendritic cells (DCs) or tumor cells, INCB024360 inhibition of IDO1 promotes T and natural killer (NK)-cell growth, increases IFN-gamma production, and reduces conversion to regulatory T (T(reg))-like cells. IDO1 induction triggers DC apoptosis, whereas INCB024360 reverses this and increases the number of CD86(high) DCs, potentially representing a novel mechanism by which IDO1 inhibition activates T cells. Furthermore, IDO1 regulation differs in DCs versus tumor cells. Consistent with its effects in vitro, administration of INCB024360 to tumor-bearing mice significantly inhibits tumor growth in a lymphocyte-dependent manner. Analysis of plasma kynurenine/tryptophan levels in patients with cancer affirms that the IDO pathway is activated in multiple tumor types. Collectively, the data suggest that selective inhibition of IDO1 may represent an attractive cancer therapeutic strategy via up-regulation of cellular immunity.[1] Malignant tumors arise, in part, because the immune system does not adequately recognize and destroy them. Expression of indoleamine-2,3-dioxygenase (IDO; IDO1), a rate-limiting enzyme in the catabolism of tryptophan into kynurenine, contributes to this immune evasion. Here we describe the effects of systemic IDO inhibition using orally active hydroxyamidine small molecule inhibitors. A single dose of INCB023843 or INCB024360 results in efficient and durable suppression of Ido1 activity in the plasma of treated mice and dogs, the former to levels seen in Ido1-deficient mice. Hydroxyamidines potently suppress tryptophan metabolism in vitro in CT26 colon carcinoma and PAN02 pancreatic carcinoma cells and in vivo in tumors and their draining lymph nodes. Repeated administration of these IDO1 inhibitors impedes tumor growth in a dose- and lymphocyte-dependent fashion and is well tolerated in efficacy and preclinical toxicology studies. Substantiating the fundamental role of tumor cell-derived IDO expression, hydroxyamidines control the growth of IDO-expressing tumors in Ido1-deficient mice. These activities can be attributed, at least partially, to the increased immunoreactivity of lymphocytes found in tumors and their draining lymph nodes and to the reduction in tumor-associated regulatory T cells. INCB024360, a potent IDO1 inhibitor with desirable pharmaceutical properties, is poised to start clinical trials in cancer patients.[2] Background and purpose: Indoleamine 2,3-dioxygenase 1 (IDO1) is emerging as an important new therapeutic target for treatment of malignant tumours characterized by dysregulated tryptophan metabolism. However, the antitumour efficacy of existing small-molecule inhibitors of IDO1 is still unsatisfactory and the underlying mechanism remains largely undefined. Hence, we discovered a novel potent small-molecule inhibitor of IDO1, LW106, and studied its antitumour effects and the underlying mechanisms in two tumour models. Experimental approach: C57BL6 mice, athymic nude mice or Ido1-/- mice were inoculated with IDO1-expressing and -nonexpressing tumour cells and treated with vehicle, epacadostat or increasing doses of LW106. Xenografted tumours, plasma, spleens and other vital organs were harvested and subjected to kynurenine/tryptophan measurement and flow cytometric, histological and immunohistochemical analyses. Key results: LW106 dose-dependently inhibited the outgrowth of xenografted tumours that were inoculated in C57BL6 mice but not nude mice or Ido1-/- mice, showing a stronger antitumour efficacy than epacadostat, an existing IDO1 inhibitor. LW106 substantially elevated intratumoural infiltration of proliferative Teff cells, while reducing recruitment of proliferative Treg cells and non-haematopoietic stromal cells such as endothelial cells and cancer-associated fibroblasts. LW106 treatment resulted in a reduced subpopulation of cancer stem cells (CSCs) in xenografted tumours in which fewer proliferative/invasive tumour cells and more apoptotic tumour cells were observed. Conclusions and implications: LW106 inhibits tumour outgrowth by limiting stroma-immune crosstalk and CSC enrichment in the tumour micro-environment. LW106 has potential as a immunotherapeutic agent for use in combination with immune checkpoint inhibitors and (or) chemotherapeutic drugs for cancer treatment.[3] Purpose: Indoleamine 2,3-dioxygenase-1 (IDO1) catalyzes the degradation of tryptophan to N-formyl-kynurenine. Overexpressed in many solid malignancies, IDO1 can promote tumor escape from host immunosurveillance. This first-in-human phase I study investigated the maximum tolerated dose, safety, pharmacokinetics, pharmacodynamics, and antitumor activity of epacadostat (INCB024360), a potent and selective inhibitor of IDO1.Experimental Design: Fifty-two patients with advanced solid malignancies were treated with epacadostat [50 mg once daily or 50, 100, 300, 400, 500, 600, or 700 mg twice daily (BID)] in a dose-escalation 3 + 3 design and evaluated in 28-day cycles. Treatment was continued until disease progression or unacceptable toxicity.Results: One dose-limiting toxicity (DLT) occurred at the dose of 300 mg BID (grade 3, radiation pneumonitis); another DLT occurred at 400 mg BID (grade 3, fatigue). The most common adverse events in >20% of patients overall were fatigue, nausea, decreased appetite, vomiting, constipation, abdominal pain, diarrhea, dyspnea, back pain, and cough. Treatment produced significant dose-dependent reductions in plasma kynurenine levels and in the plasma kynurenine/tryptophan ratio at all doses and in all patients. Near maximal changes were observed at doses of ≥100 mg BID with >80% to 90% inhibition of IDO1 achieved throughout the dosing period. Although no objective responses were detected, stable disease lasting ≥16 weeks was observed in 7 of 52 patients.Conclusions: Epacadostat was generally well tolerated, effectively normalized kynurenine levels, and produced maximal inhibition of IDO1 activity at doses of ≥100 mg BID. Studies investigating epacadostat in combination with other immunomodulatory drugs are ongoing. Clin Cancer Res; 23(13); 3269-76. [4] - Mechanism of Action: Epacadostat competitively inhibits IDO1, blocking the conversion of tryptophan to kynurenine. This restores local tryptophan levels, reversing immunosuppression in the tumor microenvironment and enhancing T cell-mediated antitumor immunity [1,2] - Clinical Development: It is investigated as an immunomodulatory agent in combination with immune checkpoint inhibitors (e.g., anti-PD-1) for the treatment of advanced solid tumors, including melanoma, non-small cell lung cancer, and renal cell carcinoma [4] Mechanism of action: Epacadostat (INCB024360) selectively inhibits IDO1, a key enzyme in the kynurenine pathway that catalyzes the rate-limiting step of tryptophan catabolism. By blocking IDO1, the drug prevents tryptophan depletion and kynurenine accumulation in the tumor microenvironment, reversing IDO1-mediated immunosuppression (e.g., T cell anergy, Treg expansion) and restoring antitumor immune responses [1,4] - Clinical design: This was a first-in-human, multicenter, open-label Phase I study to evaluate the safety, tolerability, PK, and preliminary efficacy of Epacadostat (INCB024360) in patients with advanced solid malignancies refractory to standard therapy. The primary endpoint was maximum tolerated dose (MTD) and safety; the secondary endpoint was objective response rate (ORR). No MTD was reached at 800 mg BID (the highest dose tested). Preliminary efficacy: 1 partial response (PR) in a melanoma patient (800 mg BID) and 12 patients with stable disease (SD) lasting ≥12 weeks [4] - Therapeutic potential: Literature [1] demonstrated that Epacadostat (INCB024360) enhances antitumor immunity in multiple mouse tumor models, supporting its development as an immunotherapeutic agent for cancer. Literature [4] confirmed its favorable safety profile and PK properties in humans, laying the foundation for subsequent Phase II/III studies combining Epacadostat with immune checkpoint inhibitors (e.g., anti-PD-1/PD-L1 antibodies) [1,4] - Literatures [2] (hydroxyamidine IDO inhibitors) and [3] (LW106, another IDO1 inhibitor) do not mention Epacadostat (INCB024360) and thus provide no relevant information [2,3] |
| 分子式 |
C11H13BRFN7O4S
|
|
|---|---|---|
| 分子量 |
438.23
|
|
| 精确质量 |
436.991
|
|
| 元素分析 |
C, 30.15; H, 2.99; Br, 18.23; F, 4.34; N, 22.37; O, 14.60; S, 7.32
|
|
| CAS号 |
1204669-58-8
|
|
| 相关CAS号 |
1204669-58-8 (INCB024360); 914471-09-3 (INCB14943); 1204669-37-3 (INCB024360);
|
|
| PubChem CID |
135564890
|
|
| 外观&性状 |
White to gray solid
|
|
| 密度 |
2.0±0.1 g/cm3
|
|
| 沸点 |
672.3±65.0 °C at 760 mmHg
|
|
| 闪点 |
360.4±34.3 °C
|
|
| 蒸汽压 |
0.0±2.2 mmHg at 25°C
|
|
| 折射率 |
1.742
|
|
| LogP |
3.92
|
|
| tPSA |
176Ų
|
|
| 氢键供体(HBD)数目 |
5
|
|
| 氢键受体(HBA)数目 |
11
|
|
| 可旋转键数目(RBC) |
8
|
|
| 重原子数目 |
25
|
|
| 分子复杂度/Complexity |
563
|
|
| 定义原子立体中心数目 |
0
|
|
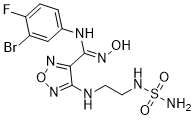
| SMILES |
BrC1=C(C([H])=C([H])C(=C1[H])/N=C(/C1C(=NON=1)N([H])C([H])([H])C([H])([H])N([H])S(N([H])[H])(=O)=O)\N([H])O[H])F
|
|
| InChi Key |
FBKMWOJEPMPVTQ-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23)
|
|
| 化学名 |
(Z)-N-(3-bromo-4-fluorophenyl)-N'-hydroxy-4-((2-(sulfamoylamino)ethyl)amino)-1,2,5-oxadiazole-3-carboximidamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.62 mg/mL (5.98 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.62 mg/mL (5.98 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.70 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.5 mg/mL (5.70 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100μL 25.0mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 5 中的溶解度: ≥ 2.5 mg/mL (5.70 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 配方 6 中的溶解度: 10%DMSO+90%PEG400: 30mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2819 mL | 11.4095 mL | 22.8191 mL | |
| 5 mM | 0.4564 mL | 2.2819 mL | 4.5638 mL | |
| 10 mM | 0.2282 mL | 1.1410 mL | 2.2819 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03361865 | Completed Has Results | Drug: Pembrolizumab Drug: Epacadostat |
UC (Urothelial Cancer) | Incyte Corporation | December 4, 2017 | Phase 3 |
| NCT03374488 | Completed Has Results | Drug: Pembrolizumab Drug: Epacadostat |
UC (Urothelial Cancer) | Incyte Corporation | December 22, 2017 | Phase 3 |
| NCT03182894 | Withdrawn | Drug: Epacadostat (INCB024360) in Combination with Pembrolizumab (MK-3475) and Azacitidine (VIDAZA) |
Metastatic Colorectal Cancer | James J Lee | September 30, 2018 | Phase 1 Phase 2 |
| NCT03516708 | Recruiting | Drug: Epacadostat Radiation: Short-course radiation |
Rectal Cancer | Washington University School of Medicine |
October 10, 2019 | Phase 1 Phase 2 |
|
|
|