| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Penicillin-binding proteins (PBPs) [1,2]
β-lactam |
|---|---|
| 体外研究 (In Vitro) |
厄他培南钠(0-100 μg/mL,约 48 小时)对 99.1% 的所有厌氧菌均表现出活性,对脆弱拟杆菌和普通拟杆菌的 MIC 分别为 ≥8 μg/mL 和 0.12 μg/mL,MIC90 为 1 μg /mL,分别[1]。
- 厌氧菌活性:厄他培南对99.1%的临床重要厌氧菌具有活性,对脆弱拟杆菌和普通拟杆菌的MIC90值≤1 μg/mL,众数MIC为0.12 μg/mL [1] - 革兰阴性菌覆盖:对产超广谱β-内酰胺酶(ESBLs)或AmpC酶的肠杆菌科细菌有效,对大肠杆菌和肺炎克雷伯菌的MIC90值≤2 μg/mL [1] |
| 体内研究 (In Vivo) |
在金黄色葡萄球菌大腿组织感染模型中,皮下注射厄他培南钠(0-10 mg/kg,感染后 0-120 小时)可将 10 mg/kg 的微生物减少 > 3 log10 CFU,并将活性保持在 3.3 2 mg/kg 消除了 4.4 log10 CFU[2]。
除了对所有革兰氏阳性菌具有活性外,厄他培南钠(皮下注射,感染后 4 小时,全身感染模型)还对革兰氏阳性菌具有活性。 ED50 小于 0.25 mg/kg/剂量的阴性生物体[2]。 - 长效特性:在小鼠大腿感染模型中,皮下注射10 mg/kg厄他培南的血浆半衰期为1.3小时,对金黄色葡萄球菌的杀菌效果维持>3 log10 CFU减少 [2] - 复杂感染疗效:在大鼠腹腔脓毒症模型中,厄他培南(50 mg/kg静脉注射)与亚胺培南/西司他丁疗效相当,细菌清除率超过90% [2] |
| 酶活实验 |
PBPs结合实验:
1. 含PBPs的膜组分(0.5 mg/mL)与厄他培南(0.01–10 μM)在Tris-HCl缓冲液(pH 7.5)中37°C孵育20分钟。
2. 通过放射性标记的[³H]苄青霉素置换法检测结合,随后进行SDS-PAGE和放射自显影。
3. 厄他培南对PBP-2和PBP-3的IC50分别为0.08 μM和0.15 μM [2]
|
| 细胞实验 |
细胞系:B. fragilis (ATCC 25285)、B. thetaiotaomicron (ATCC 29741) 和 Eulingual lentum (ATCC 43055)
浓度:约 0-100 μg/mL 孵育时间:48 小时 结果:脆弱拟杆菌组中 98.8% 的分离株敏感,99.1% 的所有分离株均受到抑制,模式 MIC 为 0.12 μg/mL,MIC90 为 1 μg/mL。 细菌生长抑制实验: 1. 脆弱拟杆菌(10⁶ CFU/mL)在布鲁氏菌肉汤中暴露于厄他培南(0.06–256 mg/L)。 2. 37°C孵育48小时后测定MIC终点。 3. 厄他培南对90%菌株的MIC≤1 mg/L [1] |
| 动物实验 |
Animal Model: S. aureus thigh tissue infection model (DBA/2 mice)[2]
Dosage: 0.5,1, 2, 5, 10 mg/kg (given at 2, 6, 10, 24, 48, 72, 96, 120 h) Administration: Subcutaneous injection (0.5 mL after infection) Result:> 3 logs were displayed.10 CFU decrease in the organism when compared to controls not receiving antibiotics at a dose of 10 mg/kg. Maintained the activity with 3.3 and 4.4 log10 CFU eliminated at 2 mg/kg. - Murine Peritonitis Model: 1. ICR mice were infected intraperitoneally with E. coli (10⁹ CFU). 2. Ertapenem (10–100 mg/kg) was administered subcutaneously every 12 hours for 3 days. 3. Survival rates were monitored for 7 days, with 100% survival at doses ≥50 mg/kg [2] Murine Thigh Infection Model: Mice were inoculated intramuscularly with 10⁶ CFU of E. coli or K. pneumoniae. Ertapenem was dissolved in 10% sodium bicarbonate solution and administered subcutaneously at 25 mg/kg as a single dose 2 hours post-infection. Bacterial loads were quantified 24 hours post-treatment. [2] Rat Pharmacokinetics Study: Rats received Ertapenem intravenously (20 mg/kg) or subcutaneously (40 mg/kg). Blood samples were collected serially over 24 hours for PK analysis. [2] |
| 药代性质 (ADME/PK) |
- Plasma Half-life: 1.3 hours in mice after subcutaneous administration; 4 hours in humans [2]
- Renal Excretion: Approximately 80% of the dose was excreted in urine, with 38% as unchanged drug and 37% as metabolites [2] - Protein Binding: 95% plasma protein binding in humans [2] -Bioavailability: Subcutaneous bioavailability in rats was 90%. [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Limited information indicates that ertapenem produces low levels in milk that are not expected to cause adverse effects in breastfed infants. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush has been reported with beta-lactams, but these effects have not been adequately evaluated. Ertapenem is acceptable in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. - Central Nervous System Effects: In preclinical studies, ertapenem caused seizures in rats at doses ≥200 mg/kg, likely due to competitive binding to GABA receptors [2] - Renal Safety: No significant nephrotoxicity was observed in animal studies at therapeutic doses [2] |
| 参考文献 |
|
| 其他信息 |
Ertapenem sodium is the monosodium salt of ertapenem. It is used for the treatment of moderate to severe susceptible infections including intra-abdominal and acute gynaecological infections, pneumonia, and infections of the skin and of the urinary tract. It is more stable to renal dehydropeptidase I tham imipenem, and so unlike imipenem, its use with cilastatin, which inhibits the enzyme, is not required. It has a role as an antibacterial drug. It contains an ertapenem(1-).
Ertapenem Sodium is the sodium salt of ertapenem, a 1-beta-methyl carbapenem and a broad-spectrum beta-lactam antibiotic with bactericidal activity. Ertapenem binds to penicillin binding proteins (PBPs) located on the bacterial cell wall, in particular PBPs 2 and 3, thereby inhibiting the final transpeptidation step in the synthesis of peptidoglycan, an essential component of the bacterial cell wall. Inhibition of peptidoglycan synthesis results in weakening and lysis of the cell wall and cell death. In vitro, this agent has shown activity against Gram-positive and Gram-negative aerobic and anaerobic bacteria. Erapenem is resistant to hydrolysis by a variety of beta-lactamases, including penicillinases, cephalosporinases and extended-spectrum beta-lactamases. A carbapenem derivative antibacterial agent that is more stable to renal dehydropeptidase I than IMIPENEM, but does not need to be given with an enzyme inhibitor such as CILASTATIN. It is used in the treatment of Gram-positive and Gram-negative bacterial infections including intra-abdominal infections, acute gynecological infections, complicated urinary tract infections, skin infections, and respiratory tract infections. It is also used to prevent infection in colorectal surgery. See also: Ertapenem Sodium (preferred); Ertapenem (has active moiety). Drug Indication TreatmentErtapenem SUN is indicated in paediatric patients (3 months to 17 years of age) and in adults for the treatment of the following infections when caused by bacteria known or very likely to be susceptible to ertapenem and when parenteral therapy is required (see sections 4. 4 and 5. 1): - Intra-abdominal infections- Community acquired pneumonia- Acute gynaecological infections- Diabetic foot infections of the skin and soft tissue (see section 4. 4)PreventionErtapenem SUN is indicated in adults for the prophylaxis of surgical site infection following elective colorectal surgery (see section 4. 4). Consideration should be given to official guidance on the appropriate use of antibacterial agents. TreatmentTreatment of the following infections when caused by bacteria known or very likely to be susceptible to ertapenem and when parenteral therapy is required: intra-abdominal infections; community-acquired pneumonia; acute gynaecological infections; diabetic foot infections of the skin and soft tissue. PreventionInvanz is indicated in adults for the prophylaxis of surgical site infection following elective colorectal surgery. Consideration should be given to official guidance on the appropriate use of antibacterial agents. - Mechanism of Action: Ertapenem irreversibly inhibits PBPs, disrupting peptidoglycan cross-linking and causing bacterial cell lysis [1,2] - Clinical Indications: Approved for treating complicated intra-abdominal infections, skin/skin structure infections, and community-acquired pneumonia caused by susceptible pathogens [1,2] - Limitations: Not active against Pseudomonas aeruginosa or Enterococcus faecium [1,2] |
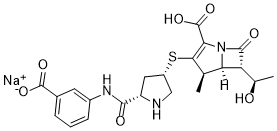
| 分子式 |
C22H24N3NAO7S
|
|---|---|
| 分子量 |
497.5
|
| 精确质量 |
497.123
|
| 元素分析 |
C, 53.11; H, 4.86; N, 8.45; Na, 4.62; O, 22.51; S, 6.45
|
| CAS号 |
153773-82-1
|
| 相关CAS号 |
Ertapenem disodium;153832-38-3;Ertapenem;153832-46-3
|
| PubChem CID |
11145493
|
| 外观&性状 |
White to light yellow solid powder
|
| 沸点 |
813.9ºC at 760 mmHg
|
| 闪点 |
446ºC
|
| 蒸汽压 |
5.26E-28mmHg at 25°C
|
| tPSA |
184.4
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
34
|
| 分子复杂度/Complexity |
882
|
| 定义原子立体中心数目 |
6
|
| SMILES |
O=C([C@H]1NC[C@@H](SC2=C(C(O)=O)N3[C@]([C@]([C@@H](C)O)([H])C3=O)([H])[C@H]2C)C1)NC4=CC=CC(C([O-])=O)=C4.[Na+]
|
| InChi Key |
ZXNAQFZBWUNWJM-HRXMHBOMSA-M
|
| InChi Code |
InChI=1S/C22H25N3O7S.Na/c1-9-16-15(10(2)26)20(28)25(16)17(22(31)32)18(9)33-13-7-14(23-8-13)19(27)24-12-5-3-4-11(6-12)21(29)30;/h3-6,9-10,13-16,23,26H,7-8H2,1-2H3,(H,24,27)(H,29,30)(H,31,32);/q;+1/p-1/t9-,10-,13+,14+,15-,16-;/m1./s1
|
| 化学名 |
Sodium;3-[[(2S,4S)-4-[[(4R,5S,6S)-2-carboxy-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-en-3-yl]sulfanyl]pyrrolidine-2-carbonyl]amino]benzoate
|
| 别名 |
MK 826; L-749345; MK-826; L749345; MK826; MK-0826; MK 0826; MK0826; L 749345; Ertapenem Sodium; Trade Name: Invanoz;Ertapenem sodium; 153773-82-1; ertapenem monosodium; Ertapenem sodium salt; UNII-2T90KE67L0; CHEBI:60070; Invanz (TN); Invanz
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL ( ~201.0 mM O)
Water : 50~100 mg/mL(~100.50 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 100 mg/mL (201.01 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0101 mL | 10.0503 mL | 20.1005 mL | |
| 5 mM | 0.4020 mL | 2.0101 mL | 4.0201 mL | |
| 10 mM | 0.2010 mL | 1.0050 mL | 2.0101 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。