| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
β-lactam; Penicillin-binding proteins (PBPs) [1,2]
|
|---|---|
| 体外研究 (In Vitro) |
- 厌氧菌活性:厄他培南对99.1%的临床重要厌氧菌具有活性,对脆弱拟杆菌和普通拟杆菌的MIC90值≤1 μg/mL,众数MIC为0.12 μg/mL [1]
- 革兰阴性菌覆盖:对产超广谱β-内酰胺酶(ESBLs)或AmpC酶的肠杆菌科细菌有效,对大肠杆菌和肺炎克雷伯菌的MIC90值≤2 μg/mL [1] 厄他培南(大约 0-100 μg/mL,48 小时)可有效对抗 99.1% 的所有厌氧菌,对脆弱拟杆菌和普通拟杆菌的 MIC ≥8 μg/mL,模式 MIC 为 0.12 μg/mL 和 MIC90分别为 1 μg/mL[1]。 |
| 体内研究 (In Vivo) |
- 长效特性:在小鼠大腿感染模型中,皮下注射10 mg/kg厄他培南的血浆半衰期为1.3小时,对金黄色葡萄球菌的杀菌效果维持>3 log10 CFU减少 [2]
- 复杂感染疗效:在大鼠腹腔脓毒症模型中,厄他培南(50 mg/kg静脉注射)与亚胺培南/西司他丁疗效相当,细菌清除率超过90% [2] 在金黄色葡萄球菌大腿组织感染模型中,厄他培南(皮下注射,0-10 mg/kg,感染后 0-120 小时)在 10 mg/kg 剂量下使微生物减少 > 3 log10 CFU,并将活性保持在 3.3 和 4.4 log10 CFU 以 2 mg/kg 消除[2]。 除了对所有革兰氏阳性菌具有活性外,厄他培南(皮下注射,感染后 4 小时,全身感染模型)还对革兰氏阴性菌具有活性ED50 小于 0.25 mg/kg/剂[2]。 |
| 酶活实验 |
PBPs结合实验:
1. 含PBPs的膜组分(0.5 mg/mL)与厄他培南(0.01–10 μM)在Tris-HCl缓冲液(pH 7.5)中37°C孵育20分钟。
2. 通过放射性标记的[³H]苄青霉素置换法检测结合,随后进行SDS-PAGE和放射自显影。
3. 厄他培南对PBP-2和PBP-3的IC50分别为0.08 μM和0.15 μM [2]
|
| 细胞实验 |
细菌生长抑制实验:
1. 脆弱拟杆菌(10⁶ CFU/mL)在布鲁氏菌肉汤中暴露于厄他培南(0.06–256 mg/L)。
2. 37°C孵育48小时后测定MIC终点。
3. 厄他培南对90%菌株的MIC≤1 mg/L [1]
细胞系:B. fragilis (ATCC 25285)、B. thetaiotaomicron (ATCC 29741) 和 Eulingual lentum (ATCC 43055) 浓度:约 0-100 μg/mL 孵育时间:48 小时 结果:脆弱拟杆菌组中 98.8% 的分离株敏感,99.1% 的所有分离株均受到抑制,模式 MIC 为 0.12 μg/mL,MIC90 为 1 μg/mL。 |
| 动物实验 |
- Murine Peritonitis Model:
1. ICR mice were infected intraperitoneally with E. coli (10⁹ CFU).
2. Ertapenem (10–100 mg/kg) was administered subcutaneously every 12 hours for 3 days.
3. Survival rates were monitored for 7 days, with 100% survival at doses ≥50 mg/kg [2]
Animal Model: S. aureus thigh tissue infection model (DBA/2 mice)[2] Dosage: 0.5,1, 2, 5, 10 mg/kg (given at 2, 6, 10, 24, 48, 72, 96, 120 h) Administration: Subcutaneous injection (0.5 mL after infection) Result: showed a reduction in organism of > 3 log10 CFU at 10 mg/kg when compared to controls not treated with antibiotics. kept up the activity at 2 mg/kg, eliminating 3.3 and 4.4 log10 CFU. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Ertapenem is almost completely absorbed following intramuscular administration, with a mean bioavailability of approximately 90%. Plasma concentrations of ertapenem are similar whether given intramuscularly or intravenously; however, the peak concentrations are lower when given via the intramuscular route. The time to reach the Cmax (Tmax) is slightly longer when given via the intramuscular route. Following daily intramuscular administration of one gram of ertapenem, the Tmax was approximately 2.3 hours. In healthy young adults who received a single 30-minute intravenous infusion of one gram of ertapenem, the Cmax was 155 µG/mL at 0.5 hours postdose. Ertapenem predominantly undergoes renal elimination, where it undergoes glomerular filtration and net tubular secretion. In healthy young adults who received one gram of IV radiolabeled ertapenem, approximately 80% of the radioactivity was recovered in urine and 10% of the radioactivity was recovered in feces. The mean percentage of the administered dose excreted in urine was 17.4% during 0-2 hours postdose, 5.4% during 4-6 hours postdose, and 2.4% during 12-24 hours postdose. Of the 80% radioactivity in urine, about 38% accounted for unchanged ertapenem and 37% accounted for its ring-opened metabolite. The apparent volume of distribution at steady state (Vss) of ertapenem is approximately 0.12 L/kg in adults, 0.2 L/kg in children three months to 12 years of age, and 0.16 L/kg in adolescents 13 to 17 years of age. Ertapenem does not accumulate. The mean plasma clearance in healthy young adults was approximately 1.8 L/hour. The mean renal clearance of intact ertapenem was 12.8 mL/min compared with a total clearance of 28.4 mL/min. Ertapenem, reconstituted with 1% lidocaine HCl injection, USP (in saline without epinephrine), is almost completely absorbed following intramuscular (IM) administration at the recommended dose of 1 g. The mean bioavailability is approximately 90%. Following 1 g daily IM administration, mean peak plasma concentrations (Cmax) are achieved in approximately 2.3 hours (Tmax). Ertapenem is highly bound to human plasma proteins, primarily albumin. In healthy young adults, the protein binding of ertapenem decreases as plasma concentrations increase, from approximately 95% bound at an approximate plasma concentration of <100 micrograms (ug)/mL to approximately 85% bound at an approximate plasma concentration of 300 ug/mL. The apparent volume of distribution at steady state (Vss) of ertapenem in adults is approximately 0.12 liter/kg, approximately 0.2 liter/kg in pediatric patients 3 months to 12 years of age and approximately 0.16 liter/kg in pediatric patients 13 to 17 years of age. The concentration of ertapenem in breast milk from 5 lactating women with pelvic infections (5 to 14 days postpartum) was measured at random time points daily for 5 consecutive days following the last 1 g dose of intravenous therapy (3-10 days of therapy). The concentration of ertapenem in breast milk within 24 hours of the last dose of therapy in all 5 women ranged from <0.13 (lower limit of quantitation) to 0.38 ug/mL; peak concentrations were not assessed. By day 5 after discontinuation of therapy, the level of ertapenem was undetectable in the breast milk of 4 women and below the lower limit of quantitation (<0.13 ug/mL) in 1 woman. For more Absorption, Distribution and Excretion (Complete) data for Ertapenem (18 total), please visit the HSDB record page. Metabolism / Metabolites In healthy young adults, unchanged ertapenem accounted for most plasma radioactivity. The major metabolite of ertapenem is the ring-opened derivative formed by dehydropeptidase I-mediated hydrolysis of the beta-lactam ring. This metabolite is pharmacologically inactive. Dehydropeptidase I (DHP-I) is found predominantly in the kidneys. Hepatic metabolism is negligible. The disposition and metabolism of ertapenem, a carbapenem antibiotic, was examined in rat, monkey and man. Sprague-Dawley rats and Rhesus monkeys were given, by intravenous administration, radiolabelled doses of ertapenem (60 and 30 mg kg(-1), respectively), and healthy normal volunteers received a single fixed dose of 1000 mg. Urine and feces were collected for determination of total radioactivity. In healthy volunteers, (14)C-ertapenem was eliminated by a combination of hydrolytic metabolism to a beta-lactam ring-opened derivative and renal excretion of unchanged drug. Approximately equal amounts were excreted as a beta-lactam ring-opened metabolite and unchanged drug (36.7 and 37.5% of dose, respectively). A secondary amide hydrolysis product accounted for about 1% of the dose in man. About 10% of the administered radioactivity was recovered in feces, which suggested that a minor fraction underwent biliary and/or intestinal excretion. In animals, a greater fraction of the dose was eliminated via metabolism; excretion of unchanged drug accounted for 17 and 5% of dose in rats and monkeys, respectively. In monkeys, the beta-lactam ring-opened and amide hydrolysis metabolites accounted for 74.8 and 7.59% of the dose, respectively, whereas in rats, these metabolites accounted for 31.9 and 20% of dose, respectively. In vitro studies with fresh rat tissue homogenates indicated that lung and kidney were the primary organs involved in mediating formation of the beta-lactam ring-opened metabolite. The specific inhibitor of dehydropeptidase-I, cilastatin, inhibited the in vivo and in vitro metabolism of ertapenem in rats, which suggested strongly that the hydrolysis of ertapenem in lung and kidney was mediated by this enzyme. Ertapenem is stable against hydrolysis by a variety of beta-lactamases, including penicillinases, and cephalosporinases and extended spectrum beta-lactamases. Ertapenem is hydrolyzed by metallo-beta-lactamases. In healthy young adults, after infusion of 1 g IV radiolabeled ertapenem, the plasma radioactivity consists predominantly (94%) of ertapenem. The major metabolite of ertapenem is the inactive ring-opened derivative formed by hydrolysis of the beta-lactam ring. Biological Half-Life The mean plasma half-life was approximately four hours in healthy young adults and adolescents and approximately 2.5 hours in children three to 12 years of age. The long half-life of ertapenem can be explained by its high protein binding. The drug has a mean plasma half-life of approximately 4 hours and may be administered once daily. The mean plasma half-life in pediatric patients 13 to 17 years of age is approximately 4 hours and approximately 2.5 hours in pediatric patients 3 months to 12 years of age. The mean plasma t(1/2) ranged from 3.8 to 4.4 hr. - Plasma Half-life: 1.3 hours in mice after subcutaneous administration; 4 hours in humans [2] - Renal Excretion: Approximately 80% of the dose was excreted in urine, with 38% as unchanged drug and 37% as metabolites [2] - Protein Binding: 95% plasma protein binding in humans [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Mild, transient, asymptomatic elevations in serum aminotransferase levels occur in about 5% of patients receiving parenteral ertapenem for 5 to 14 days. These abnormalities are usually self-limited and asymptomatic. In the limited period that it has been available, no cases of hepatitis with jaundice have been reported. Nevertheless, several instances of cholestatic jaundice arising during or shortly after therapy have been reported with other carbapenems. The latency to onset has been within 1 to 3 weeks and the pattern of enzyme elevations is usually cholestatic. Immunoallergic features can occur but autoantibodies are rare. The course is usually self-limiting, but at least one case of vanishing bile duct syndrome related to a carbapenem has been reported. Ertapenem and other carbapenems have not been linked to cases of acute liver failure. Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited information indicates that ertapenem produces low levels in milk that are not expected to cause adverse effects in breastfed infants. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush has been reported with beta-lactams, but these effects have not been adequately evaluated. Ertapenem is acceptable in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Ertapenem binds to plasma proteins in a concentration-dependent manner. It is highly bound to human plasma proteins, primarily to albumin. Protein binding is saturable at higher doses, at which the unbound fraction of the drug increases disproportionately. In healthy young adults, the protein binding of ertapenem decreased as drug plasma concentrations increased. At an approximate plasma concentration of <100 micrograms (mcg)/mL, ertapenem was 95% bound, and this percentage dropped to 85% when the plasma concentration increased to 300 mcg/mL. - Central Nervous System Effects: In preclinical studies, ertapenem caused seizures in rats at doses ≥200 mg/kg, likely due to competitive binding to GABA receptors [2] - Renal Safety: No significant nephrotoxicity was observed in animal studies at therapeutic doses [2] |
| 参考文献 |
|
| 其他信息 |
Ertapenem is meropenem in which the one of the two methyl groups attached to the amide nitrogen is replaced by hydrogen while the other is replaced by a 3-carboxyphenyl group. The sodium salt is used for the treatment of moderate to severe susceptible infections including intra-abdominal and acute gynaecological infections, pneumonia, and infections of the skin and of the urinary tract. It has a role as an antibacterial drug. It is a carbapenemcarboxylic acid and a pyrrolidinecarboxamide. It is a conjugate acid of an ertapenem(1-).
Ertapenem is a 1-β methyl-carbapenem that is structurally related to beta-lactam antibiotics. It was first authorized for use in the US in November 2001 and in Europe in April 2002. Shown to be effective against a wide range of Gram-positive and Gram-negative aerobic and anaerobic bacteria, ertapenem is used to treat various bacterial infections. Ertapenem is a Penem Antibacterial. Ertapenem is a broad spectrum carbapenem antibiotic used primarily for the treatment of aerobic gram-negative bacterial infections. Ertapenem, like other carbapenems, is associated with transient and asymptomatic elevations in serum enzymes. The carbapenems have also been linked to rare instances of clinically apparent, acute cholestatic liver injury. Ertapenem is a 1-beta-methyl carbapenem and broad-spectrum beta-lactam antibiotic with bactericidal property. Ertapenem binds to penicillin binding proteins (PBPs) located on the bacterial cell wall, in particular PBPs 2 and 3, thereby inhibiting the final transpeptidation step in the synthesis of peptidoglycan, an essential component of the bacterial cell wall. Inhibition results in a weakening and subsequent lysis of the cell wall leading to cell death of Gram-positive and Gram-negative aerobic and anaerobic pathogens. This agent is stable against hydrolysis by a variety of beta-lactamases, including penicillinases, cephalosporinases and extended-spectrum beta-lactamases. A carbapenem derivative antibacterial agent that is more stable to renal dehydropeptidase I than IMIPENEM, but does not need to be given with an enzyme inhibitor such as CILASTATIN. It is used in the treatment of Gram-positive and Gram-negative bacterial infections including intra-abdominal infections, acute gynecological infections, complicated urinary tract infections, skin infections, and respiratory tract infections. It is also used to prevent infection in colorectal surgery. See also: Ertapenem Sodium (has salt form). Drug Indication Ertapenem is indicated to treat the following moderate to severe infections caused by susceptible bacteria in adult and pediatric patients (three months of age and older): - Complicated intra-abdominal infections. - Complicated skin and skin structure infections, including diabetic foot infections without osteomyelitis. - Community-acquired pneumonia. - Complicated urinary tract infections, including pyelonephritis. - Acute pelvic infections, including postpartum endomyometritis, septic abortion and post-surgical gynecologic infections. - Acute gynecological infections. Ertapenem is also used in adults for the prophylaxis of surgical site infection following elective colorectal surgery. TreatmentErtapenem SUN is indicated in paediatric patients (3 months to 17 years of age) and in adults for the treatment of the following infections when caused by bacteria known or very likely to be susceptible to ertapenem and when parenteral therapy is required (see sections 4. 4 and 5. 1): - Intra-abdominal infections- Community acquired pneumonia- Acute gynaecological infections- Diabetic foot infections of the skin and soft tissue (see section 4. 4)PreventionErtapenem SUN is indicated in adults for the prophylaxis of surgical site infection following elective colorectal surgery (see section 4. 4). Consideration should be given to official guidance on the appropriate use of antibacterial agents. TreatmentTreatment of the following infections when caused by bacteria known or very likely to be susceptible to ertapenem and when parenteral therapy is required: intra-abdominal infections; community-acquired pneumonia; acute gynaecological infections; diabetic foot infections of the skin and soft tissue. PreventionInvanz is indicated in adults for the prophylaxis of surgical site infection following elective colorectal surgery. Consideration should be given to official guidance on the appropriate use of antibacterial agents. Mechanism of Action Ertapenem exhibits a bactericidal mode of action. It works by binding to and inhibiting bacterial penicillin-binding proteins (PBPs). In _Escherichia coli_, it has a strong affinity toward PBPs 1a, 1b, 2, 3, 4 and 5 with preferential binding to PBPs 2 and 3. Upon binding to PBPs, ertapenem inhibits bacterial cell wall synthesis by interfering with the lengthening and strengthening of the peptidoglycan portion of the cell wall, thereby inhibiting cell wall synthesis. Ertapenem is a synthetic carbapenem beta-lactam antibiotic that is structurally and pharmacologically related to imipenem and meropenem. Like meropenem but unlike imipenem, ertapenem has a methyl group at position 1 of the 5-membered ring, which confers stability against hydrolysis by dehydropeptidase 1 (DHP 1) present on the brush border of proximal renal tubular cells, and therefore does not require concomitant administration with a DHP-1 inhibitor such as cilastatin. Ertapenem has in vitro activity against Gram-positive and Gram-negative aerobic and anaerobic bacteria. The bactericidal activity of ertapenem results from the inhibition of cell wall synthesis and is mediated through ertapenem binding to penicillin binding proteins (PBPs). In Escherichia coli, it has strong affinity toward PBPs 1a, 1b, 2, 3, 4 and 5 with preference for PBPs 2 and 3. Antimicrobials are the most frequently implicated class of drugs in drug-induced seizure, with beta-lactams being the class of antimicrobials most often implicated. The seizure-inducing potential of the carbapenem subclass may be directly related to their beta-lactam ring structure. Data on individual carbapenems and seizure activity are scarce. To evaluate the available evidence on the association between carbapenem agents and seizure activity, /investigators/ conducted a literature search of the MEDLINE (1966-May 2010), EMBASE (1974-May 2010), and International Pharmaceutical Abstracts (1970-May 2010) databases. Reference citations from the retrieved articles were also reviewed. Mechanistically, seizure propensity of the beta-lactams is related to their binding to gamma-aminobutyric acid (GABA) receptors. There are numerous reports of seizure activity associated with imipenem-cilastatin, with seizure rates ranging from 3-33%. For meropenem, doripenem, and ertapenem, the seizure rate for each agent is reported as less than 1%. However, as their use increases and expands into new patient populations, the rate of seizures with these agents may increase. High-dose therapy, especially in patients with renal dysfunction, preexisting central nervous system abnormalities, or a seizure history increases the likelihood of seizure activity. - Mechanism of Action: Ertapenem irreversibly inhibits PBPs, disrupting peptidoglycan cross-linking and causing bacterial cell lysis [1,2] - Clinical Indications: Approved for treating complicated intra-abdominal infections, skin/skin structure infections, and community-acquired pneumonia caused by susceptible pathogens [1,2] - Limitations: Not active against Pseudomonas aeruginosa or Enterococcus faecium [1,2] |
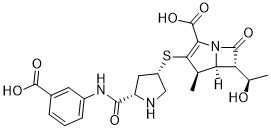
| 分子式 |
C22H25N3O7S
|
|---|---|
| 分子量 |
475.5
|
| 精确质量 |
475.141
|
| 元素分析 |
C, 55.57; H, 5.30; N, 8.84; O, 23.55; S, 6.74
|
| CAS号 |
153832-46-3
|
| 相关CAS号 |
Ertapenem sodium;153773-82-1;Ertapenem disodium;153832-38-3
|
| PubChem CID |
150610
|
| 外观&性状 |
Solid powder
|
| 密度 |
1.6±0.1 g/cm3
|
| 沸点 |
813.9±65.0 °C at 760 mmHg
|
| 熔点 |
230-234
|
| 闪点 |
446.0±34.3 °C
|
| 蒸汽压 |
0.0±3.1 mmHg at 25°C
|
| 折射率 |
1.700
|
| LogP |
-1.07
|
| tPSA |
181.57
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
893
|
| 定义原子立体中心数目 |
6
|
| SMILES |
S([C@@H]1CN[C@H](C(NC2=CC=CC(C(=O)O)=C2)=O)C1)C1=C(C(=O)O)N2C([C@]([H])([C@@H](C)O)[C@@]2([H])[C@H]1C)=O
|
| InChi Key |
JUZNIMUFDBIJCM-ANEDZVCMSA-N
|
| InChi Code |
InChI=1S/C22H25N3O7S/c1-9-16-15(10(2)26)20(28)25(16)17(22(31)32)18(9)33-13-7-14(23-8-13)19(27)24-12-5-3-4-11(6-12)21(29)30/h3-6,9-10,13-16,23,26H,7-8H2,1-2H3,(H,24,27)(H,29,30)(H,31,32)/t9-,10-,13+,14+,15-,16-/m1/s1
|
| 化学名 |
(4R,5S,6S)-3-[(3S,5S)-5-[(3-Carboxyphenyl)carbamoyl]pyrrolidin-3-yl]sulfanyl-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid
|
| 别名 |
MK 826; L-749345; MK-826; Ertapenem; 153832-46-3; (4R,5S,6S)-3-[(3S,5S)-5-[(3-carboxyphenyl)carbamoyl]pyrrolidin-3-yl]sulfanyl-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid; G32F6EID2H; CHEBI:404903; (1R,5S,6S,8R,2'S,4'S)-2-(2-(3-carboxyphenylcarbamoyl)pyrrolidin-4-ylthio)-6-(1-hydroxyethyl)-1-methylcarbapenem-3-carboxylic acid; DTXSID50165456; (4R,5S,6S)-3-((3S,5S)-5-((3-carboxyphenyl)carbamoyl)pyrrolidin-3-ylthio)-6-((R)-1-hydroxyethyl)-4-methyl-7-oxo-1-aza-bicyclo[3.2.0]hept-2-ene-2-carboxylic acid; L749345; MK826; L 749345; MK-0826; MK 0826; MK0826; Ertapenem Sodium; Trade Name: Invanoz; Invanz
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1030 mL | 10.5152 mL | 21.0305 mL | |
| 5 mM | 0.4206 mL | 2.1030 mL | 4.2061 mL | |
| 10 mM | 0.2103 mL | 1.0515 mL | 2.1030 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|