| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
Human PNP (IC50 = 1.19 nM); Mouse PNP (IC50 = 0.48 nM); Rat PNP (IC50 = 1.24 nM); Monkey PNP (IC50 = 0.66 nM); Dog PNP (IC50 = 1.57 nM)[2]
|
|---|---|
| 体外研究 (In Vitro) |
呋咯地辛 (10-30 μM) 处理 24 和 48 小时可部分减少 RPMI-8226、MOLT-4 和 5T33MM 细胞的增殖 [1]。 MM 细胞不受呋咯地辛影响(10-30 μM;24 和 48 小时;RPMI-8226、MOLT-4 和 5T33MM 细胞);然而,呋咯地辛会使 MOLT-4 细胞中活细胞的百分比降低 40%[1]。 Forodesine (BCX-1777) 可抑制各种药物、混合淋巴细胞反应 (MLR) 和白细胞介素 2 (IL-2) 刺激的人淋巴细胞增殖。植物血凝素 (PHA) 的 IC50 值小于 0.1-0.38 μM [2]。
多发性骨髓瘤(MM)是第二常见的血液系统恶性肿瘤,其特征是骨髓中恶性细胞的单克隆增殖。尽管最近在治疗策略方面取得了进展,但多发性骨髓瘤仍然无法治愈,需要新的治疗靶点。最近发现,嘌呤核苷磷酸化酶抑制剂呋咯地辛通过增加dGTP水平诱导慢性淋巴细胞白血病患者的白血病细胞凋亡。因此,我们测试了呋咯地辛是否能够通过类似的途径抑制小鼠和人类MM细胞的增殖和/或诱导凋亡。我们发现,在用呋咯地辛治疗48小时后,5T33MM和RPMI-8226 MM细胞中的dGTP略有增加,这与部分增殖抑制和有限的凋亡诱导有关。在研究导致细胞周期阻滞和凋亡的途径时,我们观察到p27、胱天蛋白酶3和BIM的上调。我们可以得出结论,呋咯地辛对MM细胞有一定的影响,但不如已知的对白血病细胞的影响那么显著。然而,呋咯地辛可能对MM中其他已建立的细胞毒性药物具有增强作用[2]。 PNP酶抑制[2] BCX-1777非常有效地抑制了人、小鼠、大鼠、猴子和狗的PNP。IC50值范围为0.48至1.57 nM(表1)。所有这些酶在3-10nM时的最大抑制效果约为90%至100%,表明BCX-1777是人类、小鼠、大鼠、猴子和狗PNP的强效抑制剂。 BCX-1777对IL-2、MLR-和PHA刺激的人T细胞淋巴增殖的抑制作用[2] 我们研究了BCX-1777抑制IL-2诱导的正常供体人PBL增殖的能力,结果如图1A所示。在dGuo(10μM)存在下,添加BCX-1777对IL-2的增殖反应产生了剂量依赖性抑制,IC50约为0.06μM。在没有dGuo的情况下,BCX-1777的IC50大于100μM。单独使用10μM的dGuo对活化淋巴细胞的增殖没有抑制作用。作为与同种异体排斥反应相关的体外实验,我们评估了BCX-1777在混合淋巴细胞反应中抑制四个供体对同种异体抗原刺激的人PBL增殖反应的能力(图1B)。如图1B所示,BCX-1777对这种反应产生了剂量依赖性的抑制作用,在0.05μM时抑制率为50%,在1μM时,抑制率为90-100%。在没有dGuo的情况下,BCX-1777在100μM以下没有明显的抑制作用。还检查了BCX-1777抑制PHA刺激的4名正常供体PBL增殖的能力(图1C)。在10μM dGuo存在下,BCX-1777对PHA刺激的细胞表现出剂量依赖性抑制作用,IC50为0.387μM。在没有dGuo的情况下,没有观察到明显的抑制作用。与未受刺激的淋巴细胞相比,用IL-2和PHA刺激的人淋巴细胞的胸苷掺入量增加了20倍以上,而用MLR刺激时,胸苷掺合量增加了10倍以上。 T细胞中的核苷酸库[2] 在BCX-1777和10μM dGuo存在的情况下,IL-2刺激的PBL显示出dGTP的积累(表2)。与对照样品相比,0.1μM BCX-1777的dGTP大约增加了4.5倍,1μM BCX-1777的dGTP增加了7.5倍。dGTP的增加与IL-2刺激的T细胞增殖的抑制平行。dGTP增加4.5倍产生52%的抑制作用,dGTP增加7.5倍产生76%的抑制作用。脱氧胞苷抑制激酶介导的dGuo转化为dGMP。为了进一步评估dGTP在T细胞抑制中的作用,在BCX-1777、dGuo和脱氧胞苷存在的情况下进行了增殖和核苷酸分析。脱氧胞苷(3μM)部分逆转了BCX-1777和dGuo对T细胞增殖的抑制作用。用BCX-1777、dGuo和dCyt孵育的T细胞的核苷酸库的测定显示,与用BCX-1777和dGuo孵育细胞相比,dGTP降低。不使用更高浓度(≥10μM)的脱氧胞苷,因为它会抑制T细胞的增殖。 IL-2刺激小鼠脾细胞增殖[2] 为了评估BCX-1777的特异性,我们评估了BCX-1777在存在和不存在dGuo(10μM)的情况下抑制IL-2诱导的小鼠脾细胞增殖的能力。在存在和不存在dGuo(10μM)的情况下,在高达100μM的BCX-1777浓度下,均未观察到对IL-2诱导的小鼠脾细胞增殖的显著抑制。与人类淋巴细胞不同,在BCX-1777(1μM)和dGuo(10μM)存在的情况下,小鼠脾细胞没有表现出dGTP的积累。 3.5.口服生物利用度和体内药理活性[2] 口服和静脉注射BCX-1777对血浆水平的影响结果如图3A所示。口服BCX-1777后迅速吸收,半小时内达到约3μM的Cmax。3小时时,血浆药物水平为1.1μM,6小时时检测不到药物。经计算,BCX-1777在小鼠体内的口服生物利用度为63%。 |
| 体内研究 (In Vivo) |
Forodesine (BCX-1777) 在小鼠体内具有出色的口服生物利用度 (63%) [2]。在小鼠中,单剂量 10 mg/kg 的 Forodesine 将 dGuo 升高至约 5 μM [2]。在人外周血淋巴细胞严重联合免疫缺陷(hu-PBL-SCID)小鼠模型中,呋咯地辛可有效延长寿命2倍以上[2]。
小鼠T细胞模型[2] 在DNFB诱导的接触性迟发型超敏反应小鼠耳水肿模型中评估了呋咯地辛/BCX-1777的体内疗效。迟发型超敏反应是由T细胞介导的。BCX-1777在30mg/kg剂量下对减轻小鼠耳水肿无效。环孢菌素(50mg/kg)用作阳性对照,耳肿胀减少58%。BCX-1777也在移植物抗宿主(GVH)诱导的小鼠脾肿大模型中进行了评估。通过注射对同种异体MHC抗原有反应并诱导全身GVHR症状的亲本T细胞,可以在成年F1小鼠中诱导实验性移植物抗宿主反应(GVHR)。GVHR类似于由T细胞对受体组织的细胞表面抗原反应介导的DTH反应。环孢菌素显著抑制了53%的GVH诱导的脾肿大,而BCX-1777在这种小鼠T细胞模型中无效。 Hu-PBL SCID小鼠模型[2] Mosier等人报道了将人PBL移植到SCID小鼠体内以构建hu-PBL SCID小鼠,并建议将其作为研究正常人类免疫功能的有用模型。最近,Sandhu等人[19]描述了一种将hu-PBLs有效植入SCID小鼠的新方案。移植到这些SCID小鼠体内的hu-PBL被证明可以通过诱导人类初级反应发挥作用。该方案的植入效率非常高,几乎100%的hu-PBL SCID小鼠在注射人PBL后不到4周内死于异种移植物抗宿主病(XGVHD)。在SCID小鼠中植入hu-PBL可诱导严重的XGVHD,并伴有体重减轻、腹泻、驼背和皮毛褶皱。这些小鼠最终因人类淋巴细胞浸润SCID小鼠组织而死亡。为了确定呋咯地辛/BCX-1777是否抑制小鼠体内人类T细胞的增殖并影响这些小鼠的寿命,从植入人类PBL前5天开始,以20mg/kg/天(b.i.d.)的剂量口服BCX-1777治疗SCID小鼠。对动物进行药物预处理以保持dGuo水平升高。按照相同的时间表继续给药,直到动物死亡。将来自三个供体的人淋巴细胞移植到三组SCID小鼠(n=10)中。实验动物(n=5)用药物治疗,对照动物(n=5)用载体(0.5%CMC)治疗。图5显示了这些实验中存活小鼠与存活天数的代表性曲线。在一项实验中,BCX-1777处理的小鼠平均寿命为30天,而对照组为15天。在另外两个实验中,BCX-1777治疗的小鼠分别存活了39天和43天,而未治疗组分别存活了17天和20天。因此,在每个实验中,BCX-1777将这些小鼠的寿命延长了2倍或更多(表3) |
| 酶活实验 |
嘌呤核苷磷酸化酶(PNP)缺乏症患者表现出选择性T细胞免疫缺陷。因此,PNP抑制剂作为潜在的T细胞选择性免疫抑制剂引起了人们的兴趣。BCX-1777是一种来自人类、小鼠、大鼠、猴子和狗等不同物种的PNP强效抑制剂,IC50值在0.48至1.57 nM之间。BCX-1777在2'-脱氧鸟苷(dGuo,3-10微M)存在下,抑制由白细胞介素-2(IL-2)、混合淋巴细胞反应(MLR)和植物血凝素(PHA)等各种试剂激活的人类淋巴细胞增殖(IC50值<0.1-0.38微M)。BCX-1777是一种比PD141955和BCX-34等其他已知PNP抑制剂强10-100倍的人类淋巴细胞增殖抑制剂。人淋巴细胞的核苷酸分析表明,BCX-1777对增殖的抑制与细胞中的dGTP水平相关[2]。
|
| 细胞实验 |
细胞增殖测定[1]
细胞类型:人 RPMI-8226、人 MOLT-4 (T-ALL) 细胞、5T33MM(多发性骨髓瘤,MM) 测试浓度: 10 μM、20 μM、30 μM 孵育时间: 24 和 48 小时 实验结果: 48 小时后(小时 )),MOLT-4细胞增殖被完全阻断,5T33MM细胞增殖减少15%。 细胞凋亡分析[1] 细胞类型:人 RPMI-8226、人 MOLT-4 (T-ALL) 细胞、5T33MM(多发性骨髓瘤,MM) 测试浓度:10 μM、20 μM、30 μM 孵育持续时间:24 小时和 48 小时(hrs) 实验结果: 细胞凋亡的诱导有限。 |
| 动物实验 |
BCX-1777 has excellent oral bioavailability (63%) in mice. At a single dose of 10 mg/kg in mice, BCX-1777 elevates dGuo to approximately 5 microM. BCX-1777 was not effective in mouse T-cell models such as delayed type hypersensitivity (DTH) and splenomegaly because mouse T-cells do not accumulate dGTP as do human T-cells. However, in the human peripheral blood lymphocyte severe combined immunodeficiency (hu-PBL-SCID) mouse model, BCX-1777 was effective in prolonging the life span 2-fold or more. This is the first known example of a PNP inhibitor that elevates dGuo in mice similar to the levels observed in PNP-deficient patients. Furthermore, these dGuo levels are also required for in vitro T-cell inhibition by BCX-1777. Thus, BCX-1777 represents a novel class of selective immunosuppressive agents that could have therapeutic utility in various T-cell disorders.[2]
Oral bioavailability and in vivo pharmacologic activity [2] Groups of four female mice (Balb/c) received a single oral or intravenous dose of the drug in sterile saline. At various time points, the mice were anesthetized (inhalation anesthesia) and bled through the retro-orbital sinus. For each time point, different sets of mice were used. The blood was centrifuged and plasma was collected and stored at −20°C until analysis. Animals were observed for 48 h for mortality and morbidity. Plasma drug levels were determined using reverse phase HPLC analysis and the quantitation limit was 0.5 μM. Deoxyguanosine levels were determined by reverse-phase HPLC and the quantitation limit for this analysis was 0.75 μM. Oral bioavailability was calculated using non-compartmental model and winnonlin software. Mouse T-cell models [2] Delayed type hypersensitivity (DTH) in mice was performed as described by Braida and Knop and Walsh et al. Briefly, mice were sensitized with 25 μl of 0.5% 2,4-dinitrofluorobenzene (DNFB) in acetone/olive oil (4:1), which was painted onto the shaved abdominal skin on days 0 and 1 (1 h after dosing). Mice were dosed once a day (q.d.) with either vehicle, Forodesine/BCX-1777 (30 mg/kg, orally) or cyclosporin A (50 mg/kg, intraperitoneally) for 6 days. On day 5, the right ears of mice were painted with 20 μl of 0.3% DNFB in acetone/olive oil (4:1), and 24 h later, the thickness of the ears were measured with a vernier caliper. The graft vs. host (GVH) reaction was evaluated in mice by measuring the degree of splenomegaly induced by the intraperitoneal injection of male hybrid mice with splenocytes from the parental strain (C57BL/6) as described by Roudebush and Bryant. Briefly, spleens were removed aseptically from C57BL/6 mice and dissociated in polypropylene mesh screens. After lysis with an ammonium chloride buffer, splenocytes were washed twice in modified HBSS and were injected i.p. (2×108 cells/ml) in hybrid mice in a volume of 0.5 ml/mouse on day 0. Hybrid mice were dosed with BCX-1777 (30 mg/kg, orally), or cyclosporin A (50 mg/kg, i.p.) for 10 days; controls received HBSS or parental spleen injections at day 0 and were dosed with vehicle either i.p. or orally. Spleens were removed and weighed and the results were recorded as a ratio of spleen weight to body weight. Hu-PBL SCID mouse model [2] The engraftment of human peripheral blood lymphocytes (hu-PBLs) in mice with severe combined immunodeficiency (SCID) was performed as described by Sandhu et al. SCID mice were pretreated 1 day prior to hu-PBL injection with a single dose of anti-ASGM1 antibodies. Anti-ASGM1 consists of rabbit polyclonal antibodies, which recognize murine NK cells, and depletes NK activity. Immediately before hu-PBL engraftment, SCID mice were irradiated. A dose of 3 Gy gamma-radiation was administered from a 137Cs source. Human lymphocytes for engraftment in SCID mice were isolated from buffy coats or from whole blood of volunteer donors. The hu-PBLs were isolated by Ficoll–Hypaque centrifugation and injected i.p. (3.0–5.0×107 PBLs/mouse) into SCID mice under sterile conditions. The experimental animals were pretreated for 5 days with Forodesine/BCX-1777 (20 mg/kg/day, b.i.d.) in 0.5% carboxymethylcellulose (CMC) to elevate the dGuo levels. Control animals were treated with 0.5% CMC. The life span of the control and experimental animals were compared. |
| 药代性质 (ADME/PK) |
Oral bioavailability and in vivo pharmacologic activity [2]
The results of oral and i.v. dosing on plasma levels of BCX-1777 are shown in Fig. 3A. BCX-1777 is absorbed rapidly after oral administration and reaches a Cmax of about 3 μM in half an hour. At 3 h, plasma drug levels were 1.1 μM and at 6 h drug was undetectable. The calculated oral bioavailability of BCX-1777 in mice was 63%. Increases in plasma dGuo levels were monitored after oral administration of BCX-1777 (10 mg/kg) in mice (Fig. 3B). dGuo increased with time and a Cmax of approximately 5 μM is achieved at 3 h. The level at 6 h is about 2.2 μM. dGuo was not detectable at 24 h. Plasma dGuo levels do not exactly parallel the drug levels. There is a delay in time at which the Cmax of dGuo is achieved (3 h) compared to the time at which the Cmax of drug is achieved (∼30 min). Plasma dGuo and drug levels in mice were monitored 3 h after oral administration of various doses of BCX-1777 (Fig. 4). BCX-1777 produced a dose-related increase in dGuo levels up to the 10 mg/kg dose. Beyond that, there were no further increases in dGuo levels. However, plasma drug levels were elevated with an increase in doses of BCX-1777 up to 100 mg/kg. |
| 参考文献 | |
| 其他信息 |
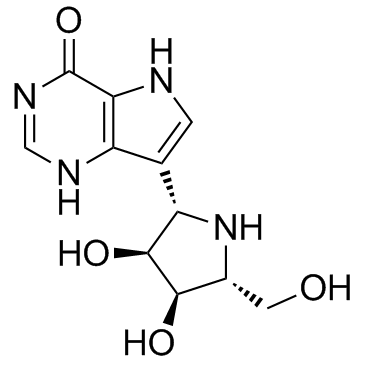
Immucillin H is a pyrrolopyrimidine and a dihydroxypyrrolidine.
Forodesine is a highly potent, orally active, rationally designed PNP inhibitor that has shown activity in preclinical studies with malignant cells and clinical utility against T-cell acute lymphoblastic leukemia and cutaneous T-cell lymphoma. Additional preliminary findings support its use for the management of some B-cell malignancies. Forodesine is a transition-state analog inhibitor of purine nucleoside phosphorylase (PNP), with potential antineoplastic activity. Upon administration, forodesine preferentially binds to and inhibits PNP, resulting in the accumulation of deoxyguanosine triphosphate and the subsequent inhibition of the enzyme ribonucleoside diphosphate reductase and DNA synthesis. This agent selectively causes apoptosis in stimulated or malignant T-lymphocytes. Drug Indication Investigated for use/treatment in lymphoma (non-hodgkin's) and leukemia (lymphoid). Patients with purine nucleoside phosphorylase (PNP) deficiency present a selective T-cell immunodeficiency. Inhibitors of PNP are, therefore, of interest as potential T-cell selective immunosuppressive agents. BCX-1777 is a potent inhibitor of PNP from various species including human, mouse, rat, monkey and dog, with IC50 values ranging from 0.48 to 1.57 nM. BCX-1777, in the presence of 2'-deoxyguanosine (dGuo, 3-10 microM), inhibits human lymphocyte proliferation activated by various agents such as interleukin-2 (IL-2), mixed lymphocyte reaction (MLR) and phytohemagglutinin (PHA) (IC50 values < 0.1-0.38 microM). BCX-1777 is a 10-100-fold more potent inhibitor of human lymphocyte proliferation than other known PNP inhibitors like PD141955 and BCX-34. Nucleotide analysis of human lymphocytes indicate that inhibition of proliferation by BCX-1777 correlates with dGTP levels in the cells. BCX-1777 has excellent oral bioavailability (63%) in mice. At a single dose of 10 mg/kg in mice, BCX-1777 elevates dGuo to approximately 5 microM. BCX-1777 was not effective in mouse T-cell models such as delayed type hypersensitivity (DTH) and splenomegaly because mouse T-cells do not accumulate dGTP as do human T-cells. However, in the human peripheral blood lymphocyte severe combined immunodeficiency (hu-PBL-SCID) mouse model, BCX-1777 was effective in prolonging the life span 2-fold or more. This is the first known example of a PNP inhibitor that elevates dGuo in mice similar to the levels observed in PNP-deficient patients. Furthermore, these dGuo levels are also required for in vitro T-cell inhibition by BCX-1777. Thus, BCX-1777 represents a novel class of selective immunosuppressive agents that could have therapeutic utility in various T-cell disorders. [2] In summary, BCX-1777 is a potent inhibitor of PNP enzyme and human T-cell proliferation. BCX-1777 is orally bioavailable in mice and can achieve maximal inhibition of PNP, thus producing elevated dGuo levels. This elevation of dGuo is found in PNP-deficient patients and proven to be necessary for T-cell inhibition. Using the hu-PBL SCID mouse model, in vivo efficacy of BCX-1777 was demonstrated. In view of the in vitro and in vivo data, we conclude that BCX-1777 is a novel, orally active, T-cell selective immunosuppressive agent that could be used for the treatment of T-cell proliferative disorders.[2] |
| 分子式 |
C11H14N4O4
|
|---|---|
| 分子量 |
266.25326
|
| 精确质量 |
266.102
|
| 元素分析 |
C, 49.62; H, 5.30; N, 21.04; O, 24.04
|
| CAS号 |
209799-67-7
|
| 相关CAS号 |
Forodesine hydrochloride;284490-13-7
|
| PubChem CID |
135409409
|
| 外观&性状 |
Light brown to gray solid
|
| 密度 |
2.01
|
| 沸点 |
613.5ºC at 760mmHg
|
| 闪点 |
324.8ºC
|
| 蒸汽压 |
6.61E-16mmHg at 25°C
|
| 折射率 |
1.894
|
| LogP |
-2.3
|
| tPSA |
134.26
|
| 氢键供体(HBD)数目 |
6
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
19
|
| 分子复杂度/Complexity |
404
|
| 定义原子立体中心数目 |
4
|
| SMILES |
O=C1C(NC=C2[C@@H]3N[C@H](CO)[C@@H](O)[C@H]3O)=C2NC=N1
|
| InChi Key |
IWKXDMQDITUYRK-KUBHLMPHSA-N
|
| InChi Code |
InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m1/s1
|
| 化学名 |
7-[(2S,3S,4R,5R)-3,4-dihydroxy-5-(hydroxymethyl)pyrrolidin-2-yl]-3,5-dihydropyrrolo[3,2-d]pyrimidin-4-one
|
| 别名 |
Forodesine; Immucillin H; 209799-67-7; Fodosine; Immucillin-H; mundesine; BCX-1777; 1,4-dideoxy-4-aza-1-(s)-(9-deazahypoxanthin-9-yl)-d-ribitol; Immucillin H; NTR 001; NTR-001; NTR001
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~375.59 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (9.39 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (9.39 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (9.39 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7559 mL | 18.7793 mL | 37.5587 mL | |
| 5 mM | 0.7512 mL | 3.7559 mL | 7.5117 mL | |
| 10 mM | 0.3756 mL | 1.8779 mL | 3.7559 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00646165 | Terminated | Drug: Forodesine | B-cell Chronic Lymphocytic Leukemia | Mundipharma Research Limited | July 2008 | Phase 1 |
| NCT00742495 | Terminated | Drug: Forodesine | Relapsed or Refractory T-cell Acute Lymphoblastic Leukaemia |
Mundipharma Research Limited | March 2009 | Phase 1 Phase 2 |
| NCT00501735 | Completed | Drug: Forodesine 200 mg | Cutaneous T-cell Lymphoma (CTCL), | BioCryst Pharmaceuticals | July 2007 | Phase 2 |
| NCT00289549 | Completed | Drug: forodesine hydrochloride (BCX-1777) |
Leukemia, Lymphocytic, Chronic | BioCryst Pharmaceuticals | June 2005 | Phase 2 |
|
|
|